Apidae
Cytogenetic data
| Subfamily | Tribe | Species | Sample local | Country(ies) | Haploid(n) | Diploid(2n) | Karyotype | Notes | Genome size (pg) | Classic cytogenetic data | Molecular cytogenetic data | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andreninae | Andrenini | Andrena dunningi | Guelph, Ontario | Canada | 0.50 | Ardila-Garcia et al. 2010 | ||||||
| Andreninae | Andrenini | Andrena togashii | Sendai, Miyagi | Japan | 3 | 6 | k= 1acrocentric + 2metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Apinae | Anthophorini | Anthophora plumipes | Matsue, Shimane | Japan | 9 | 18 | k= 1acrocentric + 8metacentric | as Anthophora acervorum villosula | C-banding | Hoshiba and Imai 1993 | ||
| Apinae | Apini | Apis cerana | Bangkok | Thailand | 0.19 | Jordan and Brosemer 1974 Ardila-Garcia et al. 2010 | ||||||
| Apinae | Apini | Apis cerana | Machida, Tokyo | Japan | 16 | 32 | all metacentric or submetacentric | as Apis cerana japonica | C-banding; G-banding | Hoshiba et al. 1981 Hoshiba and Okada 1986 Hoshiba and Imai 1993 | ||
| Apinae | Apini | Apis cerana | Mahabaleshwar | India | 16 | 32 | k= 4metacentric + 4submetacentric + 8subtelocentric | as Apis indica | Deodikar et al. 1959 | |||
| Apinae | Apini | Apis cerana | University of Peradeniya | Sri Lanka | 16 | Fahrenhorst 1977 | ||||||
| Apinae | Apini | Apis dorsata | University of Peradeniya | Sri Lanka | 16 | Fahrenhorst 1977 | ||||||
| Apinae | Apini | Apis florea | University of Peradeniya | Sri Lanka | 16 | Fahrenhorst 1977 | ||||||
| Apinae | Apini | Apis mellifera | University of Peradeniya | Sri Lanka | 16 | Fahrenhorst 1977 | ||||||
| Apinae | Apini | Apis mellifera | Itabashi-ku, Tokyo | Japan | 16 | 32 | all metacentric or submetacentric | as Apis mellifera ligustica | C-banding; G-banding | Hoshiba and Kusanagi 1978 Hoshiba 1984a Hoshiba 1984b Hoshiba and Okada 1986 Hoshiba and Imai 1993 | ||
| Apinae | Apini | Apis mellifera | Guelph, Ontario | Canada | 16 | 32 | 0.24 | HoneybeeGenomeSequencingConsortium 2006 Ardila-Garcia et al. 2010 | ||||
| Apinae | Apini | Apis mellifera | Locality not specified | Germany | 16 | FISH (ribosomal probe, TTAGG probe) | Beye and Moritz 1993 Sahara et al. 1999 | |||||
| Apinae | Apini | Apis mellifera | Banat, Timok and Syenichko–Peshterski | Serbia | 32 | as Apis mellifera carnica | G-banding | Stanimirovic et al. 2005 | ||||
| Apinae | Bombini | Bombus affinis | Locality not specified | North America | 18 | Owen 1983 | ||||||
| Apinae | Bombini | Bombus appositus | Alberta | Canada | 16 | Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus ardens | Higashimatsuyama, Saitama; Itabashi-ku, Tokyo | Japan | 18 | k= 7pseudo-acrocentric + 2acrocentric + 9metacentric | C-banding | Hoshiba and Imai 1993 | ||||
| Apinae | Bombini | Bombus ashtoni | Locality not specified | North America | 25 | as Psithyrus ashtoni | Owen 1983 | |||||
| Apinae | Bombini | Bombus bimaculatus | Guelph, Ontario | Canada | 0.34 | Ardila-Garcia et al. 2010 | ||||||
| Apinae | Bombini | Bombus bimaculatus | Ontario | Canada | 18 | Owen 1983 Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus borealis | Ontario | Canada | 16 | 32 | Owen et al. 1995 | |||||
| Apinae | Bombini | Bombus californicus | Alberta | Canada | 19 | Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus citrinus | Ontario | Canada | 26 | as Psithyrus citrinus | Owen et al. 1995 | |||||
| Apinae | Bombini | Bombus consobrinus | Fuji, Shizuoka | Japan | 19 | 38 | presence of polimorphic chromosomes with different C-banding patterns | as Bombus consobrinus wittenburgi | C-banding | Hoshiba and Imai 1993 | ||
| Apinae | Bombini | Bombus cryptarum | Alberta | Canada | 18 | As Bombus (Bombus) moderatus | Owen et al. 1995 | |||||
| Apinae | Bombini | Bombus deuteronymus | Fuji, Shizuoka | Japan | 23 | 46 | presence of polimorphic chromosomes with different C-banding patterns | as Bombus deuteronymus maruhanabachi | C-banding | Hoshiba and Imai 1993 | ||
| Apinae | Bombini | Bombus diversus | Cape Ochiishi, Hokkaido; Lake Yamanaka, Yamanashi | Japan | 18 | k= 2pseudo-acrocentric + 8acrocentric + 8metacentric | C-banding | Hoshiba and Imai 1993 | ||||
| Apinae | Bombini | Bombus ephippiatus | Locality not specified | North America | 18 | Owen 1983 | ||||||
| Apinae | Bombini | Bombus fervidus | Ontario | Canada | 18 | 36 | Owen et al. 1995 | |||||
| Apinae | Bombini | Bombus griseocollis | Ontario | Canada | 18 | Owen 1983 Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus hypocrita | Fuji, Shizuoka | Japan | 18 | k= 5pseudo-acrocentric + 2acrocentric + 11metacentric | C-banding | Hoshiba and Imai 1993 | ||||
| Apinae | Bombini | Bombus honshuensis | Lake Yamanaka, Yamanashi | Japan | 17 | 34 | k= 3pseudo-acrocentric + 3acrocentric + 11metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Apinae | Bombini | Bombus huntii | Alberta | Canada | 18 | Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus ignitus | Tsu, Mie; Tochigi Pref | Japan | 18 | k= 4pseudo-acrocentric + 3acrocentric + 11metacentric | C-banding | Hoshiba and Imai 1993 | ||||
| Apinae | Bombini | Bombus impatiens | Guelph, Ontario | Canada | 0.47 | Ardila-Garcia et al. 2010 | ||||||
| Apinae | Bombini | Bombus impatiens | Locality not specified | 0.52 | Hanrahan and Johnston 2011 | |||||||
| Apinae | Bombini | Bombus impatiens | Ontario | Canada | 18 | Owen 1983 Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus melanopygus | Locality not specified | North America | 18 | Owen 1983 | ||||||
| Apinae | Bombini | Bombus mixtus | Locality not specified | North America | 18 | Owen 1983 | ||||||
| Apinae | Bombini | Bombus morio | Locality not specified | 20 | Kerr and Silveira 1972 | |||||||
| Apinae | Bombini | Bombus nevadensis | Alberta | Canada | 17-18 | Owen 1983 | ||||||
| Apinae | Bombini | Bombus occidentalis | Locality not specified | 0.46 | Hanrahan and Johnston 2011 | |||||||
| Apinae | Bombini | Bombus occidentalis | Alberta | Canada | 18 | Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus pauloensis | Locality not specified | 20 | 40 | as Bombus (Fervidobombus) atratus | Kerr and Silveira 1972 Garófalo and Kerr 1975 | |||||
| Apinae | Bombini | Bombus pennsylvanicus | Ontario | Canada | 36 | Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus perplexus | Ontario | Canada | 12 | Owen 1983 Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus pseudobaicalensis | Cape Ochiishi and Kawakami, Hokkaido | Japan | 17 | 34 | k= 3pseudo-acrocentric + 2acrocentric + 12metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Apinae | Bombini | Bombus rufocinctus | Ontario | Canada | 18 | Owen 1983 Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus schrencki | Cape Ochiishi, Hokkaido | Japan | 17 | 34 | k= 17metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Apinae | Bombini | Bombus sitkensis | Locality not specified | North America | 18 | Owen 1983 | ||||||
| Apinae | Bombini | Bombus ternarius | Locality not specified | North America | 18 | Owen 1983 | ||||||
| Apinae | Bombini | Bombus terrestris | Tokyo | Japan | 18 | 36 | Ayabe et al. 2004 | |||||
| Apinae | Bombini | Bombus terrestris | Basel or Winterthur or Zürich | Switzerland | 18 | 0.42 - 0.64 | Gadau et al. 2001 Wilfert et al. 2006 Gregory et al. 2007 Stolle et al. 2011 | |||||
| Apinae | Bombini | Bombus terricola | Ontario | Canada | 18 | Owen 1983 Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus ussurensis | Fuji, Shizuoka | Japan | 18 | k= 3pseudo-acrocentric + 2acrocentric + 13metacentric | C-banding | Hoshiba and Imai 1993 | ||||
| Apinae | Bombini | Bombus vagans | Ontario | Canada | 18 | Owen 1983 Owen et al. 1995 | ||||||
| Apinae | Bombini | Bombus vosnesenskii | Locality not specified | North America | 18 | Owen 1983 | ||||||
| Apinae | Emphorini | Melitoma segmentaria | Viçosa, Minas Gerais | Brazil | 15 | 30 | 2k= 28pseudo-acrocentric + 2metacentric | C-banding, Ag-NOR | DAPI/CMA3 | Cristiano et al. 2014 | ||
| Apinae | Eucerini | Melissodes desponsa | Guelph, Ontario | Canada | 0.52 | Ardila-Garcia et al. 2010 | ||||||
| Apinae | Eucerini | Melissodes illata | Guelph, Ontario | Canada | 0.37 | Ardila-Garcia et al. 2010 | ||||||
| Apinae | Euglossini | Eufriesea violacea | Viçosa, Minas Gerais | Brazil | 15 | 30 | 2k= 30submetacentric | C-banding, G-banding | Gomes et al. 1998 | |||
| Apinae | Euglossini | Euglossa cordata | Cataguases, Minas Gerais | Brazil | 21 | 42 | 2k= 42submetacentric | as Euglossa carolina | C-banding | DAPI/CMA3 | Fernandes et al. 2013a | |
| Apinae | Euglossini | Euglossa cyanaspis | Panama City | Panama | 21 | k= 21submetacentric | Eltz et al. 1997 | |||||
| Apinae | Euglossini | Euglossa hyacinthina | Province Chiriqui | Panama | 20 | 40 | k= 20submetacentric | Eltz et al. 1997 | ||||
| Apinae | Euglossini | Euglossa sp. | Viçosa, Minas Gerais | Brazil | 21 | Ag-NOR | Maffei et al. 2001 | |||||
| Apinae | Euglossini | Euglossa townsendi | Viçosa, Minas Gerais | Brazil | 21 | 42 | 2k= 42submetacentric | C-banding, G-banding | DAPI/CMA3 | Fernandes et al. 2013a | ||
| Apinae | Exomalopsini | Exomalopsis auropilosa | Ribeirão Preto, São Paulo | Brazil | 9 | as Exomalopsis (Exomalopsis) aureopilosa | Kerr 1972 | |||||
| Apinae | Isepeolini | Isepeolus viperinus | Curitiba, Paraná | Brazil | 16 | Kerr 1972 | ||||||
| Apinae | Meliponini | Austroplebeia australis | Sidney | Australia | 36 | C-banding | FISH (ribosomal, repetitive, microsatellites probes) | Pereira et al. 2020 Travenzoli et al. 2022 | ||||
| Apinae | Meliponini | Camargoia nordestina | Brejo da Conceição, Piauí | Brazil | 34 | 2k= 22acrocentric + 12pseudo-acrocentric | C-banding | Rocha et al. 2003a | ||||
| Apinae | Meliponini | Celetrigona longicornis | Nova Xavantina, Mato Grosso | Brazil | 30 | 2k= 18metacentric + 12submetacentric | 0.46-0.49 | Giemsa | FISH (ribosomal, microsatellites probes) | Tavares et al. 2012 Cunha et al. 2021 Cunha et al. 2024 | ||
| Apinae | Meliponini | Cephalotrigona capitata | Piranga, Viçosa, Minas Gerais | Brazil | 34 | 2k= 18acrocentric + 16pseudo-acrocentric; 2k= 30metacentric + 4acrocentric | Giemsa, C-banding | DAPI/CMA3; FISH (ribosomal , microsatellites, satellite probes) | Rocha et al. 2003a Cunha et al. 2021 Tavares et al. 2023 Cunha et al. 2024 Vignati et al. 2025 | |||
| Apinae | Meliponini | Cephalotrigona femorata | Urbano Santos, Maranhão | Brazil | 34 | 2k= 34pseudo-acrocentric; 2k= 24metacentric + 6submetacentric + 4subtelocentric | 0.55 | C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites probes) | Tavares et al. 2012 Miranda et al. 2013 Cunha et al. 2021 Cunha et al. 2024 | ||
| Apinae | Meliponini | Cleptotrigona cubiceps | Zango and Luanda | Angola | 18 | as Lestrimelitta cubiceps | Kerr and Araujo 1957 | |||||
| Apinae | Meliponini | Dactylurina staudingeri | Ilonga | Tanzânia | 17 | Kerr 1972 | ||||||
| Apinae | Meliponini | Duckeola ghiliani | Presidente Figueiredo, Manaus, Amazonas | Brazil | 15 | 30 | 2k= 14metacentric + 12submetacentric + 4subtelocentric | as Frieseomelitta (Duckeola) ghiliani | 0.48 | Giemsa, C-banding | FISH (ribosomal, microsatellites, satellite probes) | Kerr 1972 Cunha et al. 2021 Cunha et al. 2024 Vignati et al. 2025 |
| Apinae | Meliponini | Friesella schrottkyi | Pedregulho, Cunha, São Paulo; Viçosa, Rio Paranaíba, Minas Gerais | Brazil | 17 | 34 | 2k= 10metacentric + 12submetacentric + 12subtelocentric | 0.42-0.44 | C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites, satellite probes) | Tavares et al. 2012 Elizeu et al. 2021 Vignati et al. 2025 | |
| Apinae | Meliponini | Frieseomelitta dispar | Ilhéus, Jequié, Bahia | Brazil | 15 | 30 | 2k= 6metacentric + 4acrocentric + 20pseudo-acrocentric | C-banding | DAPI/CMA3; FISH (microsatellites probes) | Carvalho and Costa 2011 Santos et al. 2018 | ||
| Apinae | Meliponini | Frieseomelitta doederleini | Santana do Seridó, Rio Grande do Norte; Canavieiras, Bahia | Brazil | 30 | 2k= 4metacentric + 4acrocentric + 22pseudo-acrocentric | C-banding | DAPI/CMA3 | Rocha et al. 2003a Nascimento et al. 2020 | |||
| Apinae | Meliponini | Frieseomelitta doederleini | Nova Ibiá, Bahia | Brazil | 30 | 2k = 4metacentric + 4acrocentric + 22pseudo-acrocentric | C-banding | DAPI/CMA3; FISH (microsatellites probes) | Santos et al. 2018 | |||
| Apinae | Meliponini | Frieseomelitta flavicornis | Iranduba, Amazonas | Brazil | 30 | 2k = 20metacentric + 10submetacentric | as Frieseomelitta sp.2 | Giemsa | FISH (ribosomal probe, microsatellite probes) | Cunha et al. 2021 Cunha et al. 2024 | ||
| Apinae | Meliponini | Frieseomelitta francoi | Cairu and Santa Inês, Bahia | Brazil | 15 | 30 | 2k= 6metacentric + 4acrocentric + 20pseudo-acrocentric | C-banding | DAPI/CMA3; FISH (microsatellite probes) | Carvalho and Costa 2011 Santos et al. 2018 | ||
| Apinae | Meliponini | Frieseomelitta languida | Arcos, Divinópolis, Minas Gerais | Brazil | 30 | 2k= 4metacentric + 4acrocentric + 22pseudo-acrocentric; 2k= 14metacentric + 14submetacentric + 2subtelocentric | C-banding | FISH (ribosomal, microsatellites probes) | Rocha et al. 2003a Rocha et al. 2003a Cunha et al. 2021 Cunha et al. 2024 | |||
| Apinae | Meliponini | Frieseomelitta longipes | Belém, Pará | Brazil | 34 | 2k= 8metacentric + 12acrocentric + 14pseudo-acrocentric | DAPI/CMA3 | Nascimento et al. 2020 | ||||
| Apinae | Meliponini | Frieseomelitta meadewaldoi | Nova Ibiá, Bahia | Brazil | 30 | 2k = 8metacentric + 4acrocentric + 18pseudo-acrocentric | C-banding | DAPI/CMA3; FISH (microsatellites probes) | Santos et al. 2018 Santos et al. 2018 | |||
| Apinae | Meliponini | Frieseomelitta portoi | Rio Branco, Acre | Brazil | 30 | 2k = 4metacentric + 26acrocentric | DAPI/CMA3 | Nascimento et al. 2020 | ||||
| Apinae | Meliponini | Frieseomelitta sp. n. | Jequié, Bahia | Brazil | 30 | 2k = 4metacentric + 4acrocentric + 22pseudo-acrocentric | 0.44 | C-banding | DAPI/CMA3; FISH (microsatellites probes) | Tavares et al. 2012 Santos et al. 2018 | ||
| Apinae | Meliponini | Frieseomelitta sp. | Brasília, Distrito Federal | Brazil | 30 | 2k = 18metacentric + 10submetacentric + 2subtelocentric | Giemsa, C-banding | FISH (ribosomal, microsatellites, satellite probes) | Cunha et al. 2021 Cunha et al. 2024 Vignati et al. 2025 | |||
| Apinae | Meliponini | Frieseomelitta trichocerata | Juína, Mato Grosso | Brazil | 30 | 2k = 6metacentric + 20acrocentric + 4pseudo-acrocentric | DAPI/CMA3 | Nascimento et al. 2020 | ||||
| Apinae | Meliponini | Frieseomelitta trichocerata | Presidente Figueiredo, Amazonas; Redenção, Pará | Brazil | 15 | 30 | 2k = 20metacentric + 10submetacentric | as Frieseomelitta sp.1 | 0.54 | Giemsa, C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites, satellite probes) | Cunha et al. 2021 Cunha et al. 2024 Andrade et al. 2024 Vignati et al. 2025 |
| Apinae | Meliponini | Frieseomelitta varia | Divinópolis, Uberlândia, Lontra, Viçosa, Minas Gerais | Brazil | 30 | 2k= 4metacentric + 4acrocentric + 22pseudo-acrocentric; 2k= 16metacentric + 12submetacentric + 2subtelocentric | Giemsa, C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites, repetitive, satellite probes) | Rocha et al. 2003a Santos et al. 2018 Pereira et al. 2020 Cunha et al. 2024 Vignati et al. 2025 | |||
| Apinae | Meliponini | Frieseomelitta varia | Ribeirão Preto, São Paulo; Bocaiúva, Minas Gerais | Brazil | 0.46-0.48 | Tavares et al. 2012 | ||||||
| Apinae | Meliponini | Geotrigona mombuca | Ribeirão Preto, São Paulo | Brazil | 15 | k= 2metacentric + 6acrocentric + 7pseudo-acrocentric | C-banding | Rocha et al. 2003a | ||||
| Apinae | Meliponini | Geotrigona subterranea | Passos, Lontra, Minas Gerais | Brazil | 34 | k= 14metacentric + 10submetacentric + 10subtelocentric | 0.83 | Giemsa | FISH (ribosomal, microsatellites, satellite probes) | Cunha et al. 2021 Cunha et al. 2024 Vignati et al. 2025 | ||
| Apinae | Meliponini | Hypotrigona gribodoi | Luanda; Massinga | Angola; Moçambique | 14 or 15 | as Hypotrigona brausi | Kerr 1972 Kerr and Silveira 1972 | |||||
| Apinae | Meliponini | Lestrimelitta limao | Divinópolis, Minas Gerais | Brazil | 28 | 2k= 6metacentric + 6acrocentric + 16pseudo-acrocentric; 2k= 18metacentric + 10submetacentric | C-banding | FISH (ribosomal, microsatellites probes) | Rocha et al. 2003a Cunha et al. 2021 Cunha et al. 2024 | |||
| Apinae | Meliponini | Lestrimelitta sp. | Domingos Martins, Espírito Santo | Brazil | 28 | 2k= 18metacentric + 10submetacentric | 0.463 | Giemsa | FISH (ribosomal, microsatellites probes) | Tavares et al. 2010a Cunha et al. 2021 Cunha et al. 2024 | ||
| Apinae | Meliponini | Lestrimelitta sulina | Lavras, Minas Gerais | Brazil | 0.53 | Cunha et al. 2021 | ||||||
| Apinae | Meliponini | Leurotrigona muelleri | Passos, Senador Mourão, Minas Gerais; Cajuru, São Paulo; Itacajá, Tocantins | Brazil | 8 | 16 | 2k= 14metacentric + 2acrocentric | 0.35 | Giemsa, C-banding | FISH (ribosomal, microsatellites, satellite probes) | Pompolo and Campos 1995 Cunha et al. 2021 Cunha et al. 2024 Vignati et al. 2025 | |
| Apinae | Meliponini | Leurotrigona muelleri | Ribeirão Preto, São Paulo | Brazil | 0.31-0.32 | Tavares et al. 2012 | ||||||
| Apinae | Meliponini | Leurotrigona pusilla | Manaus, Amazonas | Brazil | 30 | 2k= 10metacentric + 14submetacentric + 6acrocentric | C-banding | Pompolo and Campos 1995 | ||||
| Apinae | Meliponini | Melipona amazonica | Iranduba, Amazonas | Brazil | 18 | 2k= 10metacentric + 6submetacentric + 2subtelocentric | 0.31 | Giemsa | Cunha et al. 2021 | |||
| Apinae | Meliponini | Melipona asilvai | Pedra de Maria da Cruz, Minas Gerais; Santana do Seridó, Rio Grande do Norte | Brazil | 9 | 18 | 2k= 6metacentric + 10submetacentric + 2acrocentric | C-banding, Ag-NOR | DAPI/CMA3; FISH (ribosomal, microsatellites probes) | Rocha and Pompolo 1998 Rocha et al. 2002 Rocha et al. 2007 Travenzoli et al. 2019a Pereira et al. 2021a | ||
| Apinae | Meliponini | Melipona asilvai | São João do Sabugi, Paraíba | Brazil | 0.26-0.29 | Tavares et al. 2010b Tavares et al. 2012 | ||||||
| Apinae | Meliponini | Melipona bicolor | Cunha, São Paulo; Viçosa, Minas Gerais | Brazil | 9 | 18 | 2k= 6metacentric + 8submetacentric + 4acrocentric | 0.25-0.28 | C-banding | DAPI/CMA3; FISH (C0t-1, ribosomal, microsatellites, repetitive probes) | Rocha and Pompolo 1998 Rocha et al. 2002 Tavares et al. 2010b Tavares et al. 2012 Andrade-Souza et al. 2018 Travenzoli et al. 2019a Cunha et al. 2020 Pereira et al. 2021a | |
| Apinae | Meliponini | Melipona capixaba | Venda Nova do Imigrante, Espírito Santo | Brazil | 9 | 18 | high heterochromatin content species | C-banding | DAPI/CMA3 | Rocha and Pompolo 1998 Rocha et al. 2002 | ||
| Apinae | Meliponini | Melipona capixaba | Domingos Martins, Espírito Santo | Brazil | 18 | high heterochromatin content species | 1.38 | FISH (ribosomal, microsatellites probes) | Tavares et al. 2010b Travenzoli et al. 2019a Pereira et al. 2021a | |||
| Apinae | Meliponini | Melipona captiosa | Rio Branco, Acre | Brazil | 9 | 18 | high heterochromatin content species | C-banding | Rocha and Pompolo 1998 | |||
| Apinae | Meliponini | Melipona crinita | Rio Branco, Xapuri, Acre | Brazil | 9 | 18 | high heterochromatin content species | 0.73 | C-banding | DAPI/CMA3; FISH (ribosomal probe) | Rocha et al. 2002 Tavares et al. 2010b Andrade-Souza et al. 2018 | |
| Apinae | Meliponini | Melipona eburnea | Xapuri, Acre | Brazil | 1.11 | Tavares et al. 2010b | ||||||
| Apinae | Meliponini | Melipona fasciculata | São Luis, Urbano Santos, Maranhão; Belém, Altamira, Pará | Brazil | 9 | 18 | high heterochromatin content species | as Melipona compressipes in Rochaetal2002 | 0.82-0.84 | C-banding | DAPI/CMA3; FISH (C0t-1, ribosomoal, microsatellites, repetitive probes) | Rocha et al. 2002 Lopes et al. 2011 Tavares et al. 2012 Andrade-Souza et al. 2018 Travenzoli et al. 2019a Cunha et al. 2020 Pereira et al. 2021a Cunha et al. 2021 Cunha et al. 2024 |
| Apinae | Meliponini | Melipona fasciculata | Piripiri, Piauí | Brazil | as Melipona compressipes | 0.78 | Tavares et al. 2010b | |||||
| Apinae | Meliponini | Melipona favosa | Locality not specified | Suriname | 9 | 18 | 2k= 12metacentric + 4submetacentric + 2acrocentric | Suriname (referred as Surinum) | C-banding | Hoshiba 1988 Hoshiba and Imai 1993 | ||
| Apinae | Meliponini | Melipona flavolineata | Urbano Santos, Maranhão; Belém, Pará; Capixaba, Brasiléia, Xapuri, Acre | Brazil | 9 | 18 | high heterochromatin content species | 0.98 | C-banding | DAPI/CMA3; FISH (C0t-1, ribosomal, microsatellites, repetitive probes) | Lopes et al. 2011 Tavares et al. 2012 Andrade-Souza et al. 2018 Travenzoli et al. 2019a Cunha et al. 2020 Pereira et al. 2021a | |
| Apinae | Meliponini | Melipona fuliginosa | Urbano Santos, Maranhão | Brazil | 9 | 18 | high heterochromatin content species | C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites probes) | Lopes et al. 2011 Travenzoli et al. 2019a Pereira et al. 2021a | ||
| Apinae | Meliponini | Melipona fulva | Presidente Figueiredo, Amazonas | Brazil | 18 | high heterochromatin content species | Giemsa | FISH (C0t-1, ribosomal, microsatellites probes) | Cunha et al. 2020 Cunha et al. 2021 Cunha et al. 2024 | |||
| Apinae | Meliponini | Melipona fuscopilosa | Rio Branco and Xapuri, Acre | Brazil | 9 | 18 | high heterochromatin content species | 1.10 | C-banding | DAPI/CMA3; FISH (ribosomal probe) | Rocha and Pompolo 1998 Rocha et al. 2002 Tavares et al. 2010b Andrade-Souza et al. 2018 | |
| Apinae | Meliponini | Melipona grandis | Xapuri, Acre | Brazil | 18 | high heterochromatin content species | 0.95 | DAPI/CMA3; FISH (ribosomal probe) | Tavares et al. 2010b Andrade-Souza et al. 2018 | |||
| Apinae | Meliponini | Melipona interrupta | Manaus, Amazonas | Brazil | 9 | as Melipona compressipes manaosensis | Kerr 1972 | |||||
| Apinae | Meliponini | Melipona interrupta | Iranduba, Itacoatiara, Amazonas | Brazil | 18 | high heterochromatin content species | 0.80 | C-banding | DAPI/CMA3; FISH (C0t-1, ribosomal, microsatellites probes) | Travenzoli et al. 2019b Cunha et al. 2020 Cunha et al. 2021 | ||
| Apinae | Meliponini | Melipona lateralis | Presidente Figueiredo, Amazonas | Brazil | 11 | 22 | high heterochromatin content species | 0.87 | Giemsa | FISH (C0t-1, ribosomal, microsatellites probes) | Cunha et al. 2020 Cunha et al. 2021 Cunha et al. 2024 | |
| Apinae | Meliponini | Melipona nebulosa | Xapuri, Acre | Brazil | 18 | high heterochromatin content species | DAPI/CMA3; FISH (ribosomal probe) | Andrade-Souza et al. 2018 | ||||
| Apinae | Meliponini | Melipona mandacaia | Irecê, Uauá, Bahia | Brazil | 9 | 18 | 2k= 2metacentric + 14submetacentric + 2acrocentric; 2k= 4metacentric + 12submetacentric + 2acrocentric | 0.33-0.35 | C-banding | DAPI/CMA3; FISH (ribosomal probe) | Rocha et al. 2003b Tavares et al. 2010b Tavares et al. 2012 Pereira et al. 2021a | |
| Apinae | Meliponini | Melipona marginata | Caeté, Viçosa, Minas Gerais | Brazil | 9 | 18 | C-banding, Ag-NOR | DAPI/CMA3 | Rocha and Pompolo 1998 Maffei et al. 2001 Rocha et al. 2002 | |||
| Apinae | Meliponini | Melipona marginata | Cunha, São Paulo | Brazil | 0.28 | Tavares et al. 2010b | ||||||
| Apinae | Meliponini | Melipona mondury | Diogo Vasconcelos, Itamarandiba, Rio Vermelho, Viçosa, Minas Gerais; Rio Bonito, Rio de Janeiro | Brazil | 9 | 18 | high heterochromatin content species | 0.94-0.95 | C-banding | DAPI/CMA3; FISH (C0t-1, ribosomal, microsatellites, repetitive, satellite probes) | Lopes et al. 2008 Lopes et al. 2009 Tavares et al. 2012 Andrade-Souza et al. 2018 Travenzoli et al. 2019a Pereira et al. 2020 Cunha et al. 2020 Pereira et al. 2021a Vignati et al. 2025 | |
| Apinae | Meliponini | Melipona paraensis | Altamira, Pará | Brazil | 9 | 18 | high heterochromatin content species | C-banding | DAPI/CMA3; FISH (C0t-1, ribosomal, microsatellites probes) | Cunha et al. 2018 Travenzoli et al. 2019a Cunha et al. 2020 Pereira et al. 2021a | ||
| Apinae | Meliponini | Melipona paraensis | Iranduba, Amazonas | Brazil | 18 | high heterochromatin content species | as Melipona cf. rufiventris | Giemsa | FISH (ribosomal, microsatellites probes) | Cunha et al. 2021 Cunha et al. 2024 | ||
| Apinae | Meliponini | Melipona puncticollis | Altamira, Pará | Brazil | 9 | 18 | 2k= 4metacentric + 12submetacentric + 2acrocentric | C-banding | DAPI/CMA3; FISH (C0t-1, ribosomal, microsatellites, repetitive probes) | Cunha et al. 2018 Travenzoli et al. 2019a Cunha et al. 2020 Pereira et al. 2021a | ||
| Apinae | Meliponini | Melipona quadrifasciata | Pocinhos do Rio Verde, Minas Gerais; Santana de Parnaiba, São Paulo | Brazil | 9 | 18 | as Melipona quadrifasciata anthidioides | G-banding | Kerr 1972 Tambasco et al. 1979 | |||
| Apinae | Meliponini | Melipona quadrifasciata | Caeté, Viçosa, Minas Gerais; Brejo Grande, Sergipe | Brazil | 9 | 18 | 2k= 4metacentric + 12submetacentric + 2acrocentric | 0.25-0.27 | Giemsa, C-banding | DAPI/CMA3; FISH (C0t-1, ribosomal, microsatellites, repetitive, satellite probes) | Rocha and Pompolo 1998 Rocha et al. 2002 Tavares et al. 2010b Silva et al. 2012 Tavares et al. 2012 Andrade-Souza et al. 2018 Travenzoli et al. 2019a Cunha et al. 2020 Pereira et al. 2021a Pereira et al. 2021b Vignati et al. 2025 | |
| Apinae | Meliponini | Melipona quinquefasciata | Ceará; Cristalina, Luizlândia, Goiás; Brasília, Distrito Federal; Bicas, Piumhi, Januária, Caeté, Minas Gerais | Brazil | 9 | 18 | 2k= 10metacentric + 6submetacentric + 2acrocentric; 2k= 8metacentric + 6submetacentric + 4subtelocentric | up to 4 B chromosomes have been described | 0.67-0.70 | C-banding | DAPI/CMA3; FISH (C0t-1, ribosomal, microsatellites, repetitive probes) | Kerr 1972 Rocha et al. 2007 Tavares et al. 2010b Tavares et al. 2012 Silva et al. 2018 Travenzoli et al. 2019a Cunha et al. 2020 Pereira et al. 2021a Cunha et al. 2021 Cunha et al. 2024 |
| Apinae | Meliponini | Melipona rufiventris | Guimarânia, Minas Gerais | Brazil | 9 | 18 | high heterochromatin content species | 1 B chromosome has been described | 0.93 | C-banding | DAPI/CMA3; FISH (C0t-1, ribosomal, microsatellites, chromosome painting, repetitive probes) | Lopes et al. 2008 Lopes et al. 2009 Lopes et al. 2014 Travenzoli et al. 2019a Cunha et al. 2020 Pereira et al. 2021a |
| Apinae | Meliponini | Melipona scutellaris | Ilhéus, Lençóis, Encruzilhada, Bahia; Rio Claro, São Paulo; Pilões, Paraíba | Brazil | 9 | 18 | high heterochromatin content species | 1.08 | C-banding | DAPI/CMA3; FISH (C0t-1, ribosomal, U2 snDNA, microsatellites, satellite probes) | Rocha and Pompolo 1998 Rocha et al. 2002 Tavares et al. 2010b Andrade-Souza et al. 2018 Piccoli et al. 2018 Travenzoli et al. 2019a Pereira et al. 2021a Cunha et al. 2021 Pereira et al. 2021b Cunha et al. 2024 | |
| Apinae | Meliponini | Melipona seminigra | Rio Branco, Acre | Brazil | 22 | high heterochromatin content species | as Melipona seminigra abunensis | DAPI/CMA3; FISH (ribosomal probe) | Andrade-Souza et al. 2018 | |||
| Apinae | Meliponini | Melipona seminigra | Nova Xavantina, Mato Grosso | Brazil | 0.85 | Tavares et al. 2010b | ||||||
| Apinae | Meliponini | Melipona seminigra | Iranduba, Manaus, Amazonas | Brazil | 11 | 22 | high heterochromatin content species | as Melipona seminigra merrillae | 0.92 | C-banding, Ag-NOR | DAPI/CMA3; FISH (C0t-1, ribosomal, microsatellites probes) | Francini et al. 2011 Cunha et al. 2018 Cunha et al. 2020 Barbosa et al. 2021 Cunha et al. 2021 |
| Apinae | Meliponini | Melipona seminigra | Altamira, Santarém, Pará | Brazil | 11 | 22 | high heterochromatin content species | as Melipona seminigra pernigra | C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites probes) | Cunha et al. 2018 Andrade-Souza et al. 2018 Travenzoli et al. 2019a Pereira et al. 2021a Cunha et al. 2024 | |
| Apinae | Meliponini | Melipona subnitida | Mossoro, Santana do Seridó, Rio Grande do Norte | Brazil | 9 | 18 | 2k= 4metacentric + 10submetacentric + 4acrocentric | 0.27 | C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites probe) | Kerr 1972 Rocha et al. 2002 Tavares et al. 2010b Travenzoli et al. 2019a Pereira et al. 2021a | |
| Apinae | Meliponini | Melipona sp. | Brasília, Distrito Federal | Brazil | 18 | 2k= 10metacentric + 6submetacentric + 2acrocentric | Giemsa | FISH (ribosomal, microsatellites probes) | Cunha et al. 2021 Cunha et al. 2024 | |||
| Apinae | Meliponini | Meliponula becarii | Massinga | Moçambique | 17 | as Meliplebeia becarii | Kerr 1972 | |||||
| Apinae | Meliponini | Meliponula bocandei | Dande and Luanda | Angola | 18 | as Trigona (Meliponula) bocandei | Kerr and Araujo 1957 | |||||
| Apinae | Meliponini | Meliponula ferruginea | Zango and Luanda | Angola | 18 | as Trigona erithra togoensis | Kerr and Araujo 1957 | |||||
| Apinae | Meliponini | Mourella caerulea | Canguçú, Rio Grande do Sul | Brazil | 17 | 34 | k= 11acrocentric + 6pseudo-acrocentric | C-banding | Rocha et al. 2003a | |||
| Apinae | Meliponini | Nannotrigona punctata | Altamira, Pará | Brazil | 34 | 2k= 12metacentric + 6submetacentric + 16subtelocentric | Giemsa | FISH (ribosomal, microsatellites probes) | Cunha et al. 2021 Cunha et al. 2024 | |||
| Apinae | Meliponini | Nannotrigona sp. | Porto Seguro, Bahia | Brazil | 34 | 2k= 20acrocentric + 14pseudo-acrocentric | C-banding | DAPI/CMA3 | Rocha et al. 2003a | |||
| Apinae | Meliponini | Nannotrigona sp. | Xapuri, Acre | Brazil | 0.45 | Tavares et al. 2012 | ||||||
| Apinae | Meliponini | Nannotrigona testaceicornis | Viçosa, Januária, Minas Gerais | Brazil | 17 | 34 | variable among populations | 0.49-0.53 | C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites, repetitive probes) | Hoshiba and Imai 1993 Rocha et al. 2003a Tavares et al. 2012 Pereira et al. 2020 Cunha et al. 2021 Cunha et al. 2024 | |
| Apinae | Meliponini | Oxytrigona flaveola | Tangará da Serra, Mato Grosso | Brazil | 34 | 2k= 8metacentric + 8submetacentric + 16subtelocentric + 2acrocentric | as Oxytrigona cf. flaveola | DAPI/CMA3 | Krinski et al. 2010 | |||
| Apinae | Meliponini | Oxytrigona tataira | Presidente Venceslau, São Paulo | Brazil | 17 | Kerr 1972 | ||||||
| Apinae | Meliponini | Parapartamona brevipilosa | Baeza and Cosanga, Napo | Ecuador | 32 | all metacentric or submetacentric | Bravo and Arcos 1991 | |||||
| Apinae | Meliponini | Parapartamona zonata | Chiriboga and Tandayapa, Pichincha | Ecuador | 16 | 32 | all metacentric or submetacentric | Bravo and Arcos 1991 | ||||
| Apinae | Meliponini | Paratrigona lineata | Passos, Minas Gerais | Brazil | 0.49 | Cunha et al. 2021 | ||||||
| Apinae | Meliponini | Paratrigona sp. | Rio Paranaíba, Minas Gerais | Brazil | 0.44 | Cunha et al. 2021 | ||||||
| Apinae | Meliponini | Paratrigona subnuda | Ribeirão Preto, São Paulo | Brazil | 34 | 2k= 24acrocentric + 10pseudo-acrocentric | C-banding | Rocha et al. 2003a | ||||
| Apinae | Meliponini | Paratrigona subnuda | Viçosa, Minas Gerais | Brazil | 0.26 | Tavares et al. 2012 | ||||||
| Apinae | Meliponini | Partamona ailyae | Nova Xavantina, Juína, Mato Grosso | Brazil | 17 | 34 | 2k= 22metacentric + 12submetacentric | as Partamona aylae | C-banding | DAPI/CMA3; FISH (ribosomal probe) | Brito-Ribon et al. 1999 Goncalves et al. 2020 | |
| Apinae | Meliponini | Partamona auripennis | Altamira, Redenção, Pará | Brazil | 34 | 2k= 20metacentric + 12submetacentric + 2subtelocentric | C-banding | FISH (ribosomal, microsatellites probes) | Novaes et al. 2021a Tavares et al. 2023 | |||
| Apinae | Meliponini | Partamona chapadicola | Urbano Santos, Maranhão | Brazil | 17 | 34 | 2k= 24metacentric + 10submetacentric | 0.63 | C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites probes) | Tavares et al. 2012 Lopes et al. 2020 | |
| Apinae | Meliponini | Partamona cupira | Guimarânia, Minas Gerais | Brazil | 34 | 2k= 10metacentric + 22submetacentric + 2acrocentric | 1 B chromosome has been described | C-banding | DAPI/CMA3 | Marthe et al. 2010 | ||
| Apinae | Meliponini | Partamona gregaria | Novo Progresso, Pará | Brazil | 17 | 34 | 2k= 28metacentric + 6submetacentric | C-banding | FISH (ribosomal probe) | Novaes et al. 2021a | ||
| Apinae | Meliponini | Partamona helleri | Cruz das Almas, Itacaré, Jequié, Cravolândia, Macarani, Bahia; Santa Teresa, Itarana, Governador Lindemberg, Linhares, Espírito Santo; Natividade, Porciúncula, Rio de Janeiro; Araponga, Guaraciaba, Juiz de Fora, Pedra do Anta, Piranga, Porto Firme, Rio Vermelho, São Miguel do Anta, Viçosa, Ponte Nova, Antônio Prado de Minas, Carangola, Cataguases, Marliéria, Jaguaraçú, Minas Gerais; Recife, Ceará | Brazil | 17 | 34 | mostly metacentric or submetacentric | up to 7 B chromosomes have been described | 0.55-0.609 | Giemsa, C-banding, Ag-NOR | DAPI/CMA3; FISH (chromosome painting, ribosomal, repetitive, microsatellites, satellite probes) | Costa et al. 1992 Brito et al. 1997 Tosta et al. 2004 Brito et al. 2005 Tosta et al. 2007 Martins et al. 2009 Tavares et al. 2012 Martins et al. 2013 Martins et al. 2014 Tosta et al. 2014 Pereira et al. 2020 Lopes et al. 2020 Goncalves et al. 2020 Novaes et al. 2021b Tavares et al. 2023 Vignati et al. 2025 |
| Apinae | Meliponini | Partamona mulata | Cuiabá, Mato Grosso | Brazil | 17 | 34 | mostly metacentric or submetacentric | C-banding | DAPI/CMA3 | Brito-Ribon et al. 1999 | ||
| Apinae | Meliponini | Partamona nhambiquara | Nova Xavantina, Tangará da Serra, Mato Grosso | Brazil | 17 | 34 | 2k= 24metacentric + 10submetacentric | C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites probes) | Brito-Ribon et al. 1999 Lopes et al. 2020 | ||
| Apinae | Meliponini | Partamona peckolti | San Francisco de Las Pampas village, Cotopaxi province | Ecuador | 34 | C-banding | DAPI/CMA3 | Brito et al. 2003 | ||||
| Apinae | Meliponini | Partamona rustica | Januária, Lontra, Minas Gerais | Brazil | 34 | 2k= 28metacentric + 6submetacentric | 1B chromosome has been described | 0.57-0.59 | Tavares et al. 2012 Tosta et al. 2014 Novaes et al. 2021a Tavares et al. 2023 | |||
| Apinae | Meliponini | Partamona seridoensis | Sant’Ana do Seridó, Rio Grande do Norte | Brazil | 17 | 34 | mostly metacentric or submetacentric | C-banding | DAPI/CMA3; FISH (ribosomal probe) | Brito et al. 2005 | ||
| Apinae | Meliponini | Partamona sooretamae | Presidente Tancredo Neves, Uruçuca, Bahia | Brazil | 34 | 2k= 20metacentric + 14submetacentric | DAPI/CMA3; FISH (ribosomal probe) | Goncalves et al. 2020 | ||||
| Apinae | Meliponini | Partamona vicina | Nova Xavantina, Mato Grosso | Brazil | 17 | 34 | mostly metacentric or submetacentric | C-banding | DAPI/CMA3 | Brito-Ribon et al. 1999 | ||
| Apinae | Meliponini | Plebeia droryana | Santo Antônio do Jacinto, Minas Gerais | Brazil | 17 | 34 | k= 12pseudo-acrocentric + 2acrocentric + 3metacentric; 2k 18metacentric + 16submetacentric | C-banding | FISH (ribosomal, microsatellites probes) | Hoshiba and Imai 1993 Cunha et al. 2021 Cunha et al. 2024 | ||
| Apinae | Meliponini | Plebeia droryana | Viçosa, Minas Gerais | Brazil | 0.48-0.49 | Tavares et al. 2012 | ||||||
| Apinae | Meliponini | Plebeia droryana | Ribeirão Preto, São Paulo | Brazil | 0.52 | Tavares et al. 2012 | ||||||
| Apinae | Meliponini | Plebeia droryana | Iraquara, Bahia | Brazil | 34 | 2k=24metacentric + 8submetacentric + 2subtelocentric; 2k= 18metacentric + 10submetacentric + 6subtelocentric | as Plebeia aff. droryana 1 | Giemsa, C-banding | FISH (ribosomal probe) | Campos et al. 2024 | ||
| Apinae | Meliponini | Plebeia droryana | Gandu, Bahia | Brazil | 17 | 34 | 2k= 24metacentric + 6submetacentric + 4subtelocentric | as Plebeia aff. droryana 2 | Giemsa, C-banding | FISH (ribosomal probe) | Campos et al. 2024 | |
| Apinae | Meliponini | Plebeia flavocincta | Licínio de Almeida, Bahia | Brazil | 17 | 34 | 2k= 10metacentric + 18submetacentric + 6subtelocentric | as Plebeia aff. flavocincta; up to 2B chromosomes have been described | Giemsa, C-banding | FISH (ribosomal probe) | Campos et al. 2024 | |
| Apinae | Meliponini | Plebeia lucii | Viçosa, Minas Gerais | Brazil | 34 | 2k= 22pseudo-acrocentric + 12acrocentric; 2k= 6metacentric + 6submetacentric + 22subtelocentric | 0.43 | C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites probes) | Godoy et al. 2013 Tavares et al. 2012 Cunha et al. 2021 Cunha et al. 2024 | ||
| Apinae | Meliponini | Plebeia mosquito | Gandu, Bahia | Brazil | 34 | 2k= 24metacentric + 2submetacentric + 8subtelocentric | as Plebeia cf. mosquito | Giemsa, C-banding | FISH (ribosomal probe) | Campos et al. 2024 | ||
| Apinae | Meliponini | Plebeia phrynostoma | Espírito Santo | Brazil | 34 | 2k= 18pseudo-acrocentric + 10acrocentric + 6metacentric | C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites probes) | Godoy et al. 2013 Cunha et al. 2024 | |||
| Apinae | Meliponini | Plebeia poecilochroa | Linhares, Nova Almeida, Espírito Santo | Brazil | 17 | 34 | 2k= 12metacentric + 14submetacentric + 8subtelocentric | 1B chromosome has been described | Giemsa, C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites probes) | Andrade et al. 2024 | |
| Apinae | Meliponini | Plebeia sp.1 | Viçosa, Minas Gerais | Brazil | 34 | Ag-NOR | Maffei et al. 2001 | |||||
| Apinae | Meliponini | Plebeia sp. | Xapuri, Acre | Brazil | 0.43-0.44 | Tavares et al. 2012 | ||||||
| Apinae | Meliponini | Plebeia sp. nov. | Presidente Figueiredo, Amazonas | Brazil | 34 | 2k= 6metacentric + 2submetacentric + 26subtelocentric | 0.40 | Giemsa | Cunha et al. 2021 | |||
| Apinae | Meliponini | Plebeina hildebrandti | Moamba | Moçambique | 18 | as Plebeia (Plebeia) denoiti | Kerr 1972 | |||||
| Apinae | Meliponini | Ptilotrigona lurida | Manaus, Amazonas | Brazil | 11 | k= 6metacentric + 3acrocentric + 2pseudo-acrocentric | C-banding | Rocha et al. 2003a | ||||
| Apinae | Meliponini | Scaptotrigona barrocoloradensis | Although cited as “from Brazil”, this species does not occur in this Country | Brazil | 17 | 34 | k= 6pseudo-acrocentric + 8acrocentric + 3metacentric | as Trigona barrocoloralensis | C-banding | Hoshiba and Imai 1993 | ||
| Apinae | Meliponini | Scaptotrigona bipunctata | Ribeirão Preto, São Paulo | Brazil | 0.40-0.44 | Tavares et al. 2012 | ||||||
| Apinae | Meliponini | Scaptotrigona depilis | Ribeirão Preto, São Paulo | Brazil | 34 | 2k= 26acrocentric + 8pseudo-acrocentric | 0.39-0.41 | C-banding | Rocha et al. 2003a Tavares et al. 2012 | |||
| Apinae | Meliponini | Scaptotrigona postica | Ribeirão Preto, São Paulo | Brazil | 34 | 2k= 22acrocentric + 12pseudo-acrocentric | C-banding | Rocha et al. 2003a | ||||
| Apinae | Meliponini | Scaptotrigona totobi | Presidente Figueiredo, Amazonas | Brazil | 34 | 2k= 8metacentric + 6submetacentris + 20subtelocentric | as Scaptotrigona cf. polysticta | 0.52 | Giemsa | Cunha et al. 2021 | ||
| Apinae | Meliponini | Scaptotrigona tubiba | Urbano Santos, Maranhão | Brazil | 0.45 | Tavares et al. 2012 | ||||||
| Apinae | Meliponini | Scaptotrigona xanthotricha | Camacan, Valença, Bahia; Piranga, Viçosa, Minas Gerais | Brazil | 17 | 34 | variable among populations | 0.42-0.44 | C-banding, Ag-NOR | DAPI/CMA3; FISH (ribosomal, microsatellites, satellite probes) | Rocha et al. 2003a Duarte et al. 2009 Lopes et al. 2009 Tavares et al. 2012 Cunha et al. 2021 Cunha et al. 2024 Serra et al. 2023 Vignati et al. 2025 | |
| Apinae | Meliponini | Scaptotrigona sp. | São José do Sabugi, Paraíba | Brazil | 34 | 2k= 18acrocentric + 16pseudo-acrocentric | C-banding | DAPI/CMA3 | Rocha et al. 2003a | |||
| Apinae | Meliponini | Scaptotrigona sp. | Pará | Brazil | 34 | 2k= 10metacentric + 6submetacentric + 18 subtelocentric | Giemsa | FISH (ribosomal, microsatellites probes) | Cunha et al. 2021 Cunha et al. 2024 | |||
| Apinae | Meliponini | Scaura latitarsis | Ribeirão Preto, São Paulo | Brazil | 34 | 2k= 14acrocentric + 20pseudo-acrocentric | 0.43-0.44 | C-banding | Rocha et al. 2003a Tavares et al. 2012 | |||
| Apinae | Meliponini | Scaura latitarsis | Presidente Figueiredo, Amazonas | Brazil | 34 | 2k= 14submetacentric + 20subtelocentric | Giemsa | FISH (satellite probe) | Vignati et al. 2025 | |||
| Apinae | Meliponini | Schwarziana quadripunctata | Viçosa, Minas Gerais | Brazil | 34 | 2k= 18acrocentric + 16pseudo-acrocentric; 2k= 14metacentric + 8submetacentric + 12subtelocentric | 0.67 | C-banding | FISH (ribosomal, microsatellites, satellite probes) | Rocha et al. 2003a Cunha et al. 2021 Cunha et al. 2024 Vignati et al. 2025 | ||
| Apinae | Meliponini | Schwarziana sp. | Domingos Martins, Espírito Santo | Brazil | 0.65 | Tavares et al. 2012 | ||||||
| Apinae | Meliponini | Tetragona clavipes | Viçosa, Minas Gerais | Brazil | 34 | 2k= 6acrocentric + 28pseudo-acrocentric | C-banding | DAPI/CMA3 | Rocha et al. 2003a | |||
| Apinae | Meliponini | Tetragona elongata | Viçosa, Minas Gerais | Brazil | 34 | 2k= 18metacentric + 14submetacentric + 2subtelocentric | Giemsa | FISH (ribosomal, microsatellites, satellite probes) | Tavares et al. 2023 Vignati et al. 2025 | |||
| Apinae | Meliponini | Tetragonisca angustula | Locality not specified | Brazil | 17 | 34 | k= 14pseudo-acrocentric + 2acrocentric + 1metacentric | as Trigona angustula | C-banding | Hoshiba and Imai 1993 | ||
| Apinae | Meliponini | Tetragonisca angustula | Tangará da Serra, Mato Grosso; Viçosa, Mutum, Minas Gerais | Brazil | 17 | 34 | 2k= 34pseudo-acrocentric | 0.90 | C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites, satellite probes) | Rocha et al. 2003a Barth et al. 2011 Tavares et al. 2012 Pereira et al. 2020 Cunha et al. 2023 Vignati et al. 2025 | |
| Apinae | Meliponini | Tetragonisca fiebrigi | Tangará da Serra, Mato Grosso; Palotina, Paraná | Brazil | 17 | 34 | 2k= 34pseudo-acrocentric; 2k= 22metacentric + 12submetacentric | up to 7 B chromosomes have been described | C-banding | DAPI/CMA3; FISH (Chromosome painting, ribosomal, repetitive, microsatellites probes) | Barth et al. 2011 Lopes et al. 2014 Pereira et al. 2020 Pereira et al. 2020 Cunha et al. 2023 | |
| Apinae | Meliponini | Tetragonula minangkabau | Although cited as “from Brazil”, this species does not occur in this Country | Brazil | 20 | 40 | k= 7pseudo-acrocentric + 7acrocentric + 6metacentric | as Trigona minangkabau | C-banding | Hoshiba and Imai 1993 | ||
| Apinae | Meliponini | Trigona branneri | Cuiabá, Mato Grosso | Brazil | 17 | 34 | 2k= 16acrocentric + 16pseudo-acrocentric + 2metacentric | C-banding | CMA3 | Costa et al. 2004 | ||
| Apinae | Meliponini | Trigona braueri | Pedra Branca, Bahia | Brazil | 32 | 2k= 2metacentric + 4acrocentric + 26pseudo-acrocentric | as Trigona fulviventris | C-banding | DAPI/CMA3 | Domingues et al. 2005 | ||
| Apinae | Meliponini | Trigona chanchamayoensis | Cuiabá, Tangará da Serra, Mato Grosso | Brazil | 17 | 34 | 2k= 20acrocentric + 12pseudo-acrocentric + 2metacentric | C-banding | CMA3 | Costa et al. 2004 Fernandes et al. 2013b | ||
| Apinae | Meliponini | Trigona fuscipennis | Florestal, Minas Gerais | Brazil | 34 | 2k= 26metacentric + 6submetacentric + 2subtelocentric | as Trigona aff. fuscipennis | C-banding | FISH (ribosomal, microsatellites, repetitive probes) | Teixeira et al. 2023 Pereira et al. 2023 | ||
| Apinae | Meliponini | Trigona hyalinata | Cuiabá, Mato Grosso; Viçosa, Minas Gerais | Brazil | 17 | 34 | 2k= 6acrocentric + 28pseudo-acrocentric; 2k= 26metacentric + 8submetacentric | 0.53 | C-banding | FISH (ribosomal, microsatellites, repetitive probes) | Costa et al. 2004 Cunha et al. 2021 Cunha et al. 2024 Teixeira et al. 2023 | |
| Apinae | Meliponini | Trigona hypogea | Altamira, Pará | Brazil | 34 | 2k= 24metacentric + 8submetacentric + 2subtelocentric | C-banding | FISH (ribosomal, microsatellites probe) | Teixeira et al. 2023 | |||
| Apinae | Meliponini | Trigona pallens | Urbano Santos, Maranhão; Altamira, Pará | Brazil | 34 | 2k= 26metacentric + 6submetacentric + 2subtelocentric | 0.81 | C-banding | FISH (ribosomal, microsatellites, repetitive probes) | Tavares et al. 2012 Teixeira et al. 2023 Pereira et al. 2023 | ||
| Apinae | Meliponini | Trigona recursa | Luislândia, Januária, Minas Gerais; Cuiabá, Mato Grosso | Brazil | 17 | 34 | variable among populations | C-banding | CMA3; FISH (ribosomal, microsatellites, repetitive probes) | Rocha et al. 2003a Costa et al. 2004 Cunha et al. 2021 Cunha et al. 2024 Pereira et al. 2023 | ||
| Apinae | Meliponini | Trigona sp. | Cônego Marinho, Minas Gerais | Brazil | 17 | k= 2acrocentric + 15pseudo-acrocentric | as Trigona cfr. fuscipennis | C-banding | Rocha et al. 2003a | |||
| Apinae | Meliponini | Trigona sp. | Urbano Santos, Maranhão | Brazil | as Trigona fulviventris | 0.70 | Tavares et al. 2012 | |||||
| Apinae | Meliponini | Trigona spinipes | Bandeira, Januária, Janaúba, Bocaiúva, Mutum, Raul Soares, Florestal, Viçosa, Minas Gerais; Ribeirão Preto, São Paulo; Palotina, Paraná; Coaraci, Bahia; Milagres, Ceará | Brazil | 17 | 34 | variable among populations | 0.42-0.44 | Giemsa, C-banding | DAPI/CMA3; FISH (ribosomal, microsatellites, repetitive, satellite probes) | Rocha et al. 2003a Tavares et al. 2012 Barboza and Costa 2021 Tavares et al. 2021 Pereira et al. 2023 Vignati et al. 2025 | |
| Apinae | Meliponini | Trigona truculenta | Altamira, Pará | Brazil | 34 | 2k= 18metacentric + 14submetacentric + 2subtelocentric | C-banding | FISH (ribosomal, microsatellites probes) | Teixeira et al. 2023 | |||
| Apinae | Meliponini | Trigona williana | Altamira, Pará | Brazil | 34 | 2k= 26metacentric + 6submetacentric + 2subtelocentric | C-banding | FISH (ribosomal, microsatellites, repetitive probes) | Teixeira et al. 2023 Pereira et al. 2023 | |||
| Apinae | Meliponini | Trigonisca sp. | Urbano Santos, Maranhão | Brazil | 15 | 30 | k= 4metacentric + 8submetacentric + 18subtelocentric | Giemsa | FISH (ribosomal, microsatellites probes) | Cunha et al. 2021 Cunha et al. 2024 | ||
| Apinae | Xylocopini | Ceratina calcarata | Guelph, Ontario | Canada | 0.68 | Ardila-Garcia et al. 2010 | ||||||
| Apinae | Xylocopini | Ceratina dentipes | Matsue, Shimane | Japan | 17 | 34 | k= 15pseudo-acrocentric + 2metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Apinae | Xylocopini | Ceratina dupla | Guelph, Ontario | Canada | 0.59 | Ardila-Garcia et al. 2010 | ||||||
| Apinae | Xylocopini | Ceratina flavipes | Itabashi-ku, Tokyo; Matsue, Shimane; Sendai, Miyagi | Japan | 17 | 34 | k= 12pseudo-acrocentric + 5metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Apinae | Xylocopini | Ceratina japonica | Matsue, Shimane | Japan | 17 | 34 | k= 16pseudo-acrocentric + 1metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Apinae | Xylocopini | Ceratina megastigmata | Matsue, Shimane | Japan | 17 | 34 | k= 16pseudo-acrocentric + 1metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Apinae | Xylocopini | Ceratina okinawana | Iriomote, Okinawa | Japan | 17 | 34 | presence of polimorphic chromosomes with different C-banding patterns | C-banding | Hoshiba and Imai 1993 | |||
| Apinae | Xylocopini | Ceratina smaragdula | Matsue, Shimane | Japan | 14 | 28 | k= 14pseudo-acrocentric | as Pithitis smaragdula | C-banding | Hoshiba and Imai 1993 | ||
| Apinae | Xylocopini | Exoneura robusta | Dandenong Ranges of Victoria | Australia | 26 | Bousjein et al. 2019 | ||||||
| Apinae | Xylocopini | Xylocopa appendiculata | Kamakura and Sagamihara, Kanagawa | Japan | 17 | 34 | k= 13acrocentric + 4metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Apinae | Xylocopini | Xylocopa fenestrata | Ludhiana | India | 16 | 32 | as Xylocopa fenesterata | Kumbkarni 1965 | ||||
| Apinae | Xylocopini | Xylocopa virginica | Orlando, Florida | United States | as Xylocopa virginica krombeini | 0.69 | Ardila-Garcia et al. 2010 | |||||
| Collectinae | Colletini | Colletes babai | Rifu, Miyagi | Japan | 16 | 32 | k= 16pseudo-acrocentric | C-banding | Hoshiba and Imai 1993 | |||
| Collectinae | Colletini | Colletes esakii | Daisen, Tottori | Japan | 16 | k= 15pseudo-acrocentric + 1acrocentric | C-banding | Hoshiba and Imai 1993 | ||||
| Collectinae | Hylaeini | Hylaeus affinis | Lake Placid, Florida | United States | 0.64 | Ardila-Garcia et al. 2010 | ||||||
| Collectinae | Hylaeini | Hylaeus nippon | Matsue, Shimane | Japan | 8 | k= 1pseudo-acrocentric + 7metacentric | C-banding | Hoshiba and Imai 1993 | ||||
| Collectinae | Hylaeini | Hylaeus sp. 1 | Rifu, Miyagi | Japan | 18 | k= 18metacentric | C-banding | Hoshiba and Imai 1993 | ||||
| Collectinae | Hylaeini | Hylaeus sp. 2 | Rifu, Miyagi | Japan | 28 | k= 26pseudo-acrocentric + 2metacentric | C-banding | Hoshiba and Imai 1993 | ||||
| Halictinae | Augochlorini | Augochlora pura | Etna (Ithaca), New York | United States | 11 | 22 | k= 9metacentric + 2submetacentric | BrownJr and Ramberg 1985 | ||||
| Halictinae | Augochlorini | Augochlorella urania | Curitiba, Paraná | Brazil | 16 | as Augochlorella michaelis | Kerr 1972 | |||||
| Halictinae | Augochlorini | Augochloropsis metallica | Guelph, Ontario | Canada | 0.90 | Ardila-Garcia et al. 2010 | ||||||
| Halictinae | Augochlorini | Augochloropsis sparsilis | Curitiba, Paraná | Brazil | 16 | Kerr 1972 | ||||||
| Halictinae | Augochlorini | Pseudaugochlora graminea | Curitiba, Paraná | Brazil | 8 | as Pseudaugochloropsis graminea | Kerr 1972 | |||||
| Halictinae | Halictini | Agapostemon splendens | Orlando, Florida | United States | 0.66 | Ardila-Garcia et al. 2010 | ||||||
| Halictinae | Halictini | Agapostemon virescens | Ithaca, New York | United States | 17 | 34 | mostly acrocentric or subacrocentric | Ramberg et al. 1984 | ||||
| Halictinae | Halictini | Halictus aerarius | Fujisawa, Kanagawa; Rifu, Miyagi | Japan | 14 | 28 | k= 5pseudo-acrocentric + 9metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Halictinae | Halictini | Halictus ligatus | Guelph, Ontario | Canada | 0.60 | Ardila-Garcia et al. 2010 | ||||||
| Halictinae | Halictini | Halictus ligatus | Orlando, Florida | United States | 0.49 | Ardila-Garcia et al. 2010 | ||||||
| Halictinae | Halictini | Halictus poeyi | Lake Placid, Florida | United States | 0.40 | Ardila-Garcia et al. 2010 | ||||||
| Halictinae | Halictini | Lasioglossum allodalum | Rifu, Miyagi | Japan | 20 | k= 13pseudo-acrocentric + 7metacentric | C-banding | Hoshiba and Imai 1993 | ||||
| Halictinae | Halictini | Lasioglossum cooleyi | Vancouver | Canada | 18 | as Lasioglossum (Evylaeus) cooleyi | Packer and Owen 1989 | |||||
| Halictinae | Halictini | Lasioglossum duplex | Fujisawa, Kanagawa; Rifu, Miyagi | Japan | 9 | 18 | k= 9metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Halictinae | Halictini | Lasioglossum lineatulus | Ithaca, New York | United States | 12 | 24 | all metacentric or submetacentric | Dialictus lineatulus | Ramberg et al. 1984 | |||
| Halictinae | Halictini | Lasioglossum malachurum | Frankfurt | Germany | 8 | 16 | as Halictus malachurus | Noll 1931 | ||||
| Halictinae | Halictini | Lasioglossum rhytidophorus | Curitiba, Paraná | Brazil | 6 | as Lasioglossum (Dialictus) rhytidophorum | Kerr 1972 | |||||
| Halictinae | Halictini | Lasioglossum taeniolellum | Sagamihara, Kanagawa | Japan | 16 | 32 | k= 7pseudo-acrocentric + 3acrocentric + 6metacentric | C-banding | Hoshiba and Imai 1993 | |||
| Halictinae | Halictini | Lasioglossum zephyrum | Ithaca, New York | United States | 13 | 26 | all metacentric or submetacentric | Dialictus zephyrus | Ramberg et al. 1984 | |||
| Megachilinae | Anthidiini | Loyolanthidium notatum | Orlando, Florida | United States | as Anthidiellum notatum rufimaculatum | 0.48 | Ardila-Garcia et al. 2010 | |||||
| Megachilinae | Megachilini | Megachile ainu | Matsue, Shimane | Japan | 16 | k= 16pseudo-acrocentric | C-banding | Hoshiba and Imai 1993 | ||||
| Megachilinae | Megachilini | Megachile albitarsis | Orlando, Florida | United States | 0.80 | Ardila-Garcia et al. 2010 | ||||||
| Megachilinae | Megachilini | Megachile pseudomonticola | Matsue, Shimane | Japan | 16 | k= 7pseudo-acrocentric + 9metacentric | C-banding | Hoshiba and Imai 1993 | ||||
| Megachilinae | Megachilini | Megachile rotundata | Guelph, Ontario | Canada | 0.83 | Ardila-Garcia et al. 2010 | ||||||
| Megachilinae | Megachilini | Megachile sculpturalis | Matsue, Shimane; Higashimatsuyama, Saitama | Japan | 16 | k= 4pseudo-acrocentric + 12metacentric | as Chalicodoma sculpturalis | C-banding | Hoshiba and Imai 1993 | |||
| Megachilinae | Megachilini | Megachile spissula | Sendai, Miyagi | Japan | 16 | k= 14pseudo-acrocentric + 2metacentric | as Chalicodoma spissula | C-banding | Hoshiba and Imai 1993 | |||
| Megachilinae | Osmini | Osmia cornifrons | Matsue, Shimane | Japan | 16 | k= 7pseudo-acrocentric + 9metacentric | C-banding | Hoshiba and Imai 1993 | ||||
| Megachilinae | Osmini | Osmia cornuta | Locality not specified | 16 | 32 | Armbruster 1913 Kerr and Laidlaw 1956 | ||||||
| Megachilinae | Osmini | Osmia lignaria | Matsue, Shimane | Japan | 16 | k= 16pseudo-acrocentric | as Osmia lignaria propinqua | C-banding | Hoshiba and Imai 1993 | |||
| Megachilinae | Osmini | Osmia taurus | Matsue, Shimane | Japan | 16 | k= 16pseudo-acrocentric | C-banding | Hoshiba and Imai 1993 |
Chromosome classification: Levan et al. (1964): metacentric (m), submetacentric (sm), subtelocentric (st), or acrocentric (a). Imai (1991): metacentric (M) or acrocentric (A), with a variety of forms depending on the position of the heterochromatin on the karyotype.
You can download this data as csv.
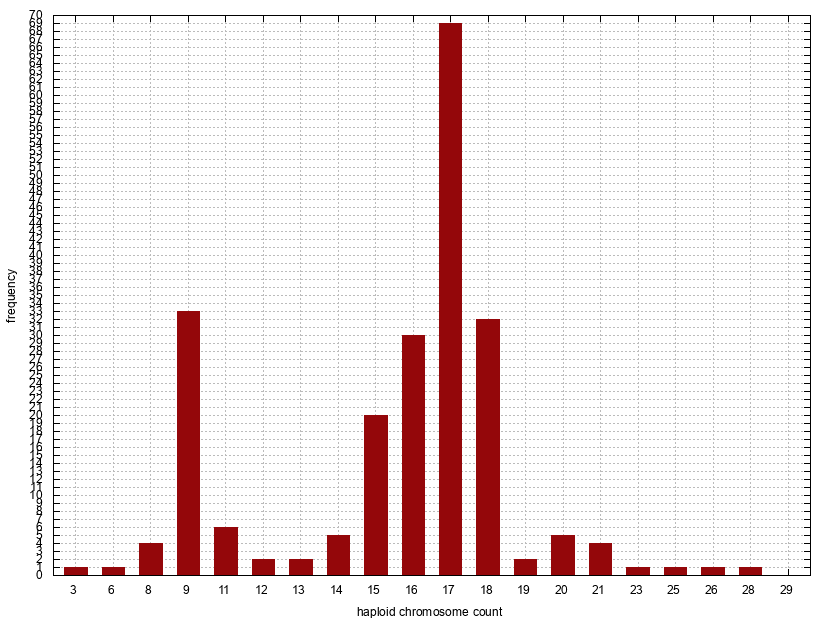
Histogram
Bibliography
Fernandes, A., Barth, A., Sampaio, W.S. Caracterização citogenética da espécie Trigona chanchamayoensis (Hymenoptera, Apidae, Meliponini) encontrada no cerrado brasileiro. Evolução e Conservação da Biodiversidade, 4 : pp 63-67. 2013b. DOI: [10.7902/ecb.v4i2.55 https://doi.org/10.7902/ecb.v4i2.55]
Rocha, M.P., Pompolo, S.G. Karyotypes and heterochromatin variation (C-bands) in Melipona species (Hymenoptera, Apidae, Meliponinae). Genetics and Molecular Biology, 21 : pp . 1998. DOI: [10.1590/S1415-47571998000100008 https://doi.org/10.1590/S1415-47571998000100008]
Gomes, L.F., Brito, R.M., Pompolo, S.G., Campos, L.A.O., Peruquetti, R.C. Karyotype and C-and G-banding patterns of Eufriesea violacea (Hymenoptera, Apidae, Euglossinae). Hereditas, 128 : pp 73-76. 1998. DOI: [10.1111/j.1601-5223.1998.00073.x https://doi.org/10.1111/j.1601-5223.1998.00073.x]
Bousjein, N.S., Zahed, M.A., & Oryan, S. The first contribution to karyotyping of the Australian allodapine bee Exoneura robusta. Journal of Apicultural Research, : pp 1-3. 2019. DOI: [10.1080/00218839.2019.1637225 https://doi.org/10.1080/00218839.2019.1637225]
Tambasco, A.J., Giannoni, M.A., Azevedo-Moreira, L.M. Analyses of G-bands in chromosomes of the Melipona quadrifasciata anthidioides Lepeletier (Hymenoptera, Apidae, Meliponinae). Cytologia, 44 : pp 21-27. 1979. DOI: [10.1508/cytologia.44.21 https://doi.org/10.1508/cytologia.44.21]
Noll, J. Untersuchungen über die Zeugung und Staatenbildung des Halictus malachurus Kirby. Zeitschrift für Morphologie und Ökologie der Tiere, 23 : pp 285-368. 1931. DOI: [10.1007/BF00446352 https://doi.org/10.1007/BF00446352]
Hoshiba, H., Okada, I. G-Banding analyses of male chromosomes in Apis cerana and A. mellifera ligustica. Apidologie, 17 : pp 101-106. 1986. DOI: [10.1051/apido:19860203 https://doi.org/10.1051/apido:19860203]
Hoshiba, H., Kusanagi, A. Karyological study of honeybee. Journal of Apicultural Research, 17 : pp 105-109. 1978. DOI: [10.1080/00218839.1978.11099913 https://doi.org/10.1080/00218839.1978.11099913]
Santos, J.M.D., Diniz, D., Rodrigues, T.A.S., Cioffi, M.D.B., Waldschmidt, A.M. Heterochromatin distribution and chromosomal mapping of microsatellite repeats in the genome of Frieseomelitta stingless bees (Hymenoptera: Apidae: Meliponini). Florida Entomologist, 101 : pp 33-39. 2018. DOI: [10.1653/024.101.0107 https://doi.org/10.1653/024.101.0107]
Francini, I.B., Gross, M.C., Nunes-Silva, C.G., Carvalho-Zilse, G.A. Cytogenetic analysis of the Amazon stingless bee Melipona seminigra merrillae reveals different chromosome number for the genus. Scientia Agricola, 68 : pp 592-593. 2011. DOI: [10.1590/S0103-90162011000500012 https://doi.org/10.1590/S0103-90162011000500012]
Tosta, V.C., Marthe, J.B., Tavares, M.G., Fernandes-Salomão, T.M., Pompolo, S.G., Recco-Pimentel, S.M., Perfectti, F., Campos, L.A.O., Camacho, J.P.M. Possible introgression of B chromosomes between bee species (genus Partamona). Cytogenetic and Genome Research, 144 : pp 220-226. 2014. DOI: [10.1159/000370171 https://doi.org/10.1159/000370171]
Andrade-Souza, V., Duarte, O.M.P., Martins, C.C.C., Santos, I.S., Costa, M.G.C., Costa, M.A. Comparative molecular cytogenetics in Melipona Illiger species (Hymenoptera, Apidae). Sociobiology, 65 : pp 696-705. 2018. DOI: [10.13102/sociobiology.v65i4.3480 https://doi.org/10.13102/sociobiology.v65i4.3480]
Tosta, V.C., Tavares, M.G., Fernandes-Salomão, T.M., Barros, E.G., Campos, L.A.O., Camacho, J.P.M. Development of a SCAR marker for the analysis of B chromosome presence in Partamona helleri (Hymenoptera, Apidae). Cytogenetic and Genome Research, 116 : pp 127-129. 2007. DOI: [10.1159/000097430 https://doi.org/10.1159/000097430]
Tavares, M.G., Carvalho, C.R., Soares, F.A.F., Campos, L.A.O. Genome size diversity in stingless bees (Hymenoptera: Apidae, Meliponini). Apidologie, 43 : pp 731-736. 2012. DOI: [10.1007/s13592-012-0145-x https://doi.org/10.1007/s13592-012-0145-x]
Carvalho, A.F., Costa, M.A. Cytogenetic characterization of two species of Frieseomelitta Ihering, 1912 (Hymenoptera, Apidae, Meliponini). Genetics and Molecular Biology, 34 : pp 237-239. 2011. DOI: [10.1590/S1415-47572011005000010 https://doi.org/10.1590/S1415-47572011005000010]
Brito, R.M., Caixeiro, A.P.D.A., Pompolo, S.G., Azevedo, G.G. Cytogenetic data of Partamona peckolti (Hymenoptera, Apidae, Meliponini) by C banding and fluorochrome staining with DA/CMA3 and DA/DAPI. Genetics and Molecular Biology, 26 : pp 53-57. 2003. DOI: [10.1590/S1415-47572003000100009 https://doi.org/10.1590/S1415-47572003000100009]
Serra, R.S., Campos, L.A.O., Serrão, J.E. Evidence that workers recognize unfertilized queenâlaid eggs for male production in stingless bees. Acta Zoologica, 104 : pp 118-124. 2023. DOI: [10.1111/azo.12401 https://doi.org/10.1111/azo.12401]
Cunha, M.S., Campos, L.A.O., Lopes, D.M. Insights into the heterochromatin evolution in the genus Melipona (Apidae: Meliponini). Insectes Sociaux, 67 : pp 391-398. 2020. DOI: [10.1007/s00040-020-00773-6 https://doi.org/10.1007/s00040-020-00773-6]
Kerr, W.E., Silveira, Z.V.D. Karyotypic evolution of bees and corresponding taxonomic implications. Evolution, 26 : pp 197-202. 1972. DOI: [10.1111/j.1558-5646.1972.tb00187.x https://doi.org/10.1111/j.1558-5646.1972.tb00187.x]
Lopes, D.M., Fernandes, A., Diniz, D., Scudeler, P.E.S., Foresti, F., Campos, L.A.O. Similarity of heterochromatic regions in the stingless bees (Hymenoptera: Meliponini) revealed by chromosome painting. Caryologia, 67 : pp 222-226. 2014. DOI: [10.1080/0144235X.2014.974349 https://doi.org/10.1080/0144235X.2014.974349]
Hoshiba, H., Imai, H. Chromosome evolution of bees and wasps (Hymenoptera, Apocrita) on the basis of C-banding pattern analyses. Japanese Journal of Entomology, 61 : pp 465-492. 1993
Miranda, R.V., Fernandes, A., Lopes, D.M. Karyotype description of Cephalotrigona femorata Smith (Hymenoptera: Apidae) and the C-banding pattern as a specific marker for Cephalotrigona. Sociobiology, 60 : pp 125-127. 2013. DOI: [10.13102/sociobiology.v60i1.125-127 https://doi.org/10.13102/sociobiology.v60i1.125-127]
Eltz, T., Schmid, M., Roubik, D.W. Haploid karyotypes of two species of orchid bees (Hymenoptera: Apidae, Euglossini). Journal of the Kansas Entomological Society, 70 : pp 142144. 1997
Duarte, O.M.P., Martins, C.C.C., Waldschmidt, A.M., Costa, M.A. Occurrence of multiple nucleolus organizer regions and intraspecific karyotype variation in Scaptotrigona xanthotricha Moure (Hymenoptera, Meliponini). Genetics and Molecular Research, 8 : pp 831-839. 2009. DOI: [10.4238/vol8-3gmr598 https://doi.org/10.4238/vol8-3gmr598]
Lopes, D.M., Tranvenzoli, N.M., Fernandes, A., Campos, L.A.O. Different levels of chromatin condensation in Partamona chapadicola and Partamona nhambiquara (Hymenoptera, Apidae). Cytogenetic and Genome Research, : pp . 2020. DOI: [10.1159/000507835 https://doi.org/10.1159/000507835]
Tavares, M.G., Carvalho, C.R., Soares, F.A.F., Fernandes, A. Detection of diploid males in a natural colony of the cleptobiotic bee Lestrimelitta sp (Hymenoptera, Apidae). Genetics and Molecular Biology, 33 : pp 491-493. 2010a. DOI: [10.1590/S1415-47572010000300019 https://doi.org/10.1590/S1415-47572010000300019]
Vignati, Z.B.M., Teixeira, G.A., Cunha, M.S., Pereira, J.A., Lopes, D.M. Cytogenomics of Frieseomelitta varia (Hymenoptera: Apidae) and the sharing of a satellite DNA family in several Neotropical Meliponini genera. Genes, 16 : pp 86. 2025. DOI: [10.3390/genes16010086 https://doi.org/10.3390/genes16010086]
Costa, K.F., Brito, R.M., Miyazawa, C.S. Karyotypic description of four species of Trigona (Jurine, 1807) (Hymenoptera, Apidae, Meliponini) from the state of Mato Grosso, Brazil. Genetics and Molecular Biology, 27 : pp 187-190. 2004. DOI: [10.1590/S1415-47572004000200010 https://doi.org/10.1590/S1415-47572004000200010]
Armbruster, L. Chromosomenverhaltnisse bei der Spermatogenese solitarer Apiden (Osmia cornuta Latr.). Arch Zellforsch, 11 : pp 242-326. 1913
Novaes, C.M., Cunha, M.S., Werneck, H.A., Fernandes, A., Campos, L.A.O., Lopes, D.M. Chromosome evolution in the genus Partamona (Apidae: Meliponini), with comments on B chromosome origin. Cytogenetic and Genome Research, 160 : pp 520-528. 2021a. DOI: [10.1159/000520552 https://doi.org/10.1159/000520552]
Deodikar, G.B., Thakar, C.V., Shah, P.N. Cyto-genetic studies in Indian honeybees. I. Somatic chromosome complement in Apis indica and its bearing on evolution and phylogeny. Proceedings of the Indian Academy of Sciences Section B, 49 : pp 194-206. 1959
Rocha, M.P., Cruz, M.P., Fernandes, A., Waldschmidt, A.M., Silva-Junior, J.C., Pompolo, S.G. Longitudinal differentiation in Melipona mandacaia (Hymenoptera, Meliponini) chromosomes. Hereditas, 138 : pp 133-137. 2003b. DOI: [10.1034/j.1601-5223.2003.01699.x https://doi.org/10.1034/j.1601-5223.2003.01699.x]
Brown Jr, W.L., Ramberg, F.B. Karyotype of Augochlora pura (Hymenoptera: Halictidae). Entomological News, 96 : pp 161-162. 1985
Tavares, M.G., Oliveira, E.R., Elizeu, A.M., Novaes, C.M., Travenzoli, N.M., Lopes, D.M. Comparative molecular cytogenetics in five species of stingless bees (Hymenoptera, Apidae). Zoologischer Anzeiger, 302 : pp 37-42. 2023. DOI: [10.1016/j.jcz.2022.11.008 https://doi.org/10.1016/j.jcz.2022.11.008]
Kerr, W.E., Laidlaw, H.H. General genetics of bees. Advances in Genetics, 8 : pp 109-153. 1956. DOI: [10.1016/S0065-2660(08)60501-5 https://doi.org/10.1016/S0065-2660(08)60501-5]
Piccoli, M.C.A., Bardella, V.B., Cabral-de-Mello, D.C. Repetitive DNAs in Melipona scutellaris (Hymenoptera: Apidae: Meliponidae): chromosomal distribution and test of multiple heterochromatin amplification in the genus. Apidologie, 49 : pp 497-504. 2018. DOI: [10.1007/s13592-018-0577-z https://doi.org/10.1007/s13592-018-0577-z]
Kumbkarni, C.G. Cytological studies in Hymenoptera: Part II: Cytology of parthenogenesis in the carpenter-bee, Xylocopa fenesterata Fabre. Cytologia, 30 : pp 222-228. 1965. DOI: [10.1508/cytologia.30.222 https://doi.org/10.1508/cytologia.30.222]
Fahrenhorst, H. Nachweis ubereinstimmender Chromosomen-Zahlen (n = 16) bei allen 4 Apis-Arten. Apidologie, 8 : pp 89-100. 1977. DOI: [10.1051/apido:19770107 https://doi.org/10.1051/apido:19770107]
Packer, L., Owen, R.E. Notes on the biology of Lasioglossum (Evylaevs) cooleyi (Crawford), an eusocial Halictine bee (Hymenoptera: Halictidae). The Canadian Entomologist, 121 : pp 431-438. 1989. DOI: [10.4039/Ent121431-6 https://doi.org/10.4039/Ent121431-6]
Cristiano, M.P., Simões, T.G., Lopes, D.M., Pompolo, S.G. Cytogenetics of Melitoma segmentaria (Fabricius, 1804) (Hymenoptera, Apidae) reveals differences in the characteristics of heterochromatin in bees. Comparative Cytogenetics, 8 : pp 223-231. 2014. DOI: [10.3897/CompCytogen.v8i3.7510 https://doi.org/10.3897/CompCytogen.v8i3.7510]
Cunha, M.S., Garcia, M.V.B., Campos, L.A.O., Lopes, D.M. Cytotaxonomy and karyotype evolution in Neotropical Meliponini (Hymenoptera: Apidae) inferred by chromosomal mapping of 18S rDNA and five microsatellites. Journal of Apicultural Research, 63 : pp 208-218. 2024. DOI: [10.1080/00218839.2023.2179228 https://doi.org/10.1080/00218839.2023.2179228]
Pereira, J.A., Salomão, T.M.F., Lopes, D.M. Different repetitive DNA sequences make up heterochromatin in Meliponini. Apidologie, 51 : pp 855-860. 2020. DOI: [10.1007/s13592-020-00766-1 https://doi.org/10.1007/s13592-020-00766-1]
Kerr, W.E., Araújo, V.P. Contribuição ao estudo citológico dos Apoidea. Garcia da Orta, 5 : pp 431-433. 1957
Cunha, M.S., Soares, F.A.F., Clarindo, W.R., Campos, L.A.O., Lopes, D.M. Robertsonian rearrangements in Neotropical Meliponini karyotype evolution (Hymenoptera: Apidae: Meliponini). Insect Molecular Biology, 30 : pp 379-389. 2021. DOI: [10.1111/imb.12702 https://doi.org/10.1111/imb.12702]
Godoy, D.C., Ferreira, R.P., Lopes, D.M. Chromosomal variation and cytogenetics of Plebeia lucii and P. phrynostoma (Hymenoptera: Apidae). Florida Entomologist, 96 : pp 1559-1566. 2013. DOI: [10.1653/024.096.0439 https://doi.org/10.1653/024.096.0439]
Beye, M., Moritz, R.F.A. In situ hybridization of rDNA on chromosomes of the honeybee, Apis mellifera L.. Experientia, 49 : pp 337-338. 1993. DOI: [10.1007/BF01923416 https://doi.org/10.1007/BF01923416]
Lopes, D.M., Pompolo, S.G., Campos, L.A.O., Tavares, M.G. Cytogenetic characterization of Melipona rufiventris Lepeletier 1836 and Melipona mondury Smith 1863 (Hymenoptera, Apidae) by C banding and fluorochromes staining. Genetics and Molecular Biology, 31 : pp 49-52. 2008. DOI: [10.1590/S1415-47572008000100010 https://doi.org/10.1590/S1415-47572008000100010]
Pereira, J.A., Travenzoli, N.M., Oliveira, M.P., Werneck, H.A., Fernandes-Salomão, T.M., Lopes, D.M. Molecular cytogenetics in the study of repetitive sequences helping to understand the evolution of heterochromatin in Melipona (Hymenoptera, Meliponini). Genetica, 149 : pp 55-62. 2021a. DOI: [10.1007/s10709-020-00111-5 https://doi.org/10.1007/s10709-020-00111-5]
Jordan J.R., Brosemer R.W. Characterization of DNA from three bee species. Journal of Insect Physiology, 20 : pp 25132520. 1974. DOI: [10.1016/0022-1910(74)90035-3 https://doi.org/10.1016/0022-1910(74)90035-3]
Barboza, V.P., Costa, M.A. Cytogenetic Analysis in Trigona spinipes Fabricius (Hymenoptera, Meliponina) reveals intraspecific variation. Neotropical Entomology, 50 : pp 846-849. 2021. DOI: [10.1007/s13744-021-00853-7 https://doi.org/10.1007/s13744-021-00853-7]
Gadau, J., Gerloff, C.U., Krüger, N., Chan, H., Schmid-Hempel, P., Wille, A., Page Jr, R.E. A linkage analysis of sex determination in Bombus terrestris (L.) (Hymenoptera: Apidae). Heredity, 87 : pp 234-242. 2001. DOI: [10.1046/j.1365-2540.2001.00919.x https://doi.org/10.1046/j.1365-2540.2001.00919.x]
Lopes, D.M., Fernandes, A., Praça-Fontes, M.M., Werneck, H.D.A., Resende, H.C., Campos, L.A.O. Cytogenetics of three Melipona species (Hymenoptera, Apidae, Meliponini). Sociobiology, 58 : pp 185-194. 2011
Barth, A., Fernandes, A., Pompolo, S.G., Costa, M.A. Occurrence of B chromosomes in Tetragonisca Latreille, 1811 (Hymenoptera, Apidae, Meliponini): a new contribution to the cytotaxonomy of the genus. Genetics and Molecular Biology, 34 : pp 77-79. 2011. DOI: [10.1590/S1415-47572010005000100 https://doi.org/10.1590/S1415-47572010005000100]
Fernandes, A., Werneck, H.A., Pompolo, S.G., Lopes, D.M. Evidence of separate karyotype evolutionary pathway in Euglossa orchid bees by cytogenetic analyses. Anais da Academia Brasileira de Ciências, 85 : pp 937-944. 2013a. DOI: [10.1590/S0001-37652013005000050 https://doi.org/10.1590/S0001-37652013005000050]
Stanimirovic, Z., Stevanovic, J., Andjelkovic, M. Chromosomal diversity in Apis mellifera carnica from Serbia. Apidologie, 36 : pp 31â42. 2005. DOI: [10.1051/apido:2004067 https://doi.org/10.1051/apido:2004067]
Travenzoli, N.M., Cunha, M.S., Teixeira, L.V., Brito, R.M., Oldroyd, B., Campos, L.A.O., Lopes, D.M. Cytogenetic characterization of Austroplebeia australis: evolutionary hints from a stingless bee outside the Neotropical region. Apidologie, 53 : pp 64. 2022. DOI: [10.1007/s13592-022-00969-8 https://doi.org/10.1007/s13592-022-00969-8]
Marthe, J.D.B., Pompolo, S.G., Campos, L.A.O., Salomão,T.M.F., Tavares, M.G. Cytogenetic characterization of Partamona cupira (Hymenoptera, Apidae) by fluorochromes. Genetics and Molecular Biology, 33 : pp 253-255. 2010. DOI: [10.1590/S1415-47572010005000029 https://doi.org/10.1590/S1415-47572010005000029]
Cunha, M.S., Travenzoli, N.M., Ferreira, R.D.P., Cassinela, E.K., Silva, H.B.D., Oliveira, F.P.M., Salomão, T.M.F., Lopes, D.M. Comparative cytogenetics in three Melipona species (Hymenoptera: Apidae) with two divergent heterochromatic patterns. Genetics and Molecular Biology, 41 : pp 806-813. 2018. DOI: [10.1590/1678-4685-gmb-2017-0330 https://doi.org/10.1590/1678-4685-gmb-2017-0330]
Pereira, J.A., Cabral-de-Mello, D.C., Lopes, D.M. The Satellite DNAs populating the genome of Trigona hyalinata and the sharing of a highly abundant satDNA in Trigona Genus. Genes, 14 : pp 418. 2023. DOI: [10.3390/genes14020418 https://doi.org/10.3390/genes14020418]
Hoshiba, H. The C-banding analysis of the diploid male and female honeybee (Apis mellifera). Proceedings of the Japan Academy Series B, 60 : pp 238-240. 1984b. DOI: [10.2183/pjab.60.238 https://doi.org/10.2183/pjab.60.238]
Hoshiba, H. Karyological analysis of a stingless bee, Melipona favosa (Apidae, Hymenoptera). Cytologia, 53 : pp 153-156. 1988. DOI: [10.1508/cytologia.53.153 https://doi.org/10.1508/cytologia.53.153]
Tavares, M.G., Carvalho, C.R., Soares, F.A.F. Genome size variation in Melipona species (Hymenoptera: Apidae) and sub-grouping by their DNA content. Apidologie, 41 : pp 636-642. 2010b. DOI: [10.1051/apido/20010023 https://doi.org/10.1051/apido/20010023]
Hanrahan, S.J., Johnston, J.S. New genome size estimates of 134 species of arthropods. Chromosome Research, 19 : pp 809-823. 2011. DOI: [10.1007/s10577-011-9231-6 https://doi.org/10.1007/s10577-011-9231-6]
Brito-Ribon, R.M., Miyazawa, C.S., Pompolo, S.G. First karyotype characterization of four species of Partamona (Friese, 1980) (Hymenoptera, Apidae, Meliponinae) in Mato Grosso State, Brazil. Cytobios, 100 : pp 19-26. 1999
Pereira, J.A., Milani, D., Ferretti, A.B.S.M., Bardella, V.B., Cabral-de-Mello, D.C., Lopes, D.M. The extensive amplification of heterochromatin in Melipona bees revealed by high throughput genomic and chromosomal analysis. Chromosoma, 130 : pp 251-262. 2021b. DOI: [10.1007/s00412-021-00764-x https://doi.org/10.1007/s00412-021-00764-x]
Martins, C.C.C., Waldschmidt, A.M., Costa, M.A. Unprecedented record of ten novel B chromosomes in the stingless bee Partamona helleri (Apidae, Meliponini). Apidologie, 45 : pp 431-439. 2014. DOI: [10.1007/s13592-013-0257-y https://doi.org/10.1007/s13592-013-0257-y]
Garófalo, C.A., Kerr, W.E. Sex determination in bees. I. Balance between femaleness and maleness genes in Bombus atratus Franklin (Hymenoptera, Apidae). Genetica, 45 : pp 203-209. 1975. DOI: [10.1007/BF01517196 https://doi.org/10.1007/BF01517196]
Ardila-Garcia, A.M., Umphrey, G.J., Gregory, T.R. An expansion of the genome size dataset for the insect order Hymenoptera, with a first test of parasitism and eusociality as possible constraints. Insect Molecular Biology, 19 : pp 337-346. 2010. DOI: [10.1111/j.1365-2583.2010.00992.x https://doi.org/10.1111/j.1365-2583.2010.00992.x]
Cunha, M.S., Novaes, C.M., Pereira, J.A., Capoco, M.M., Fernandes-Salomao, T.M., Lopes, D.M. Supernumerary B chromosomes of Tetragonisca fiebrigi share repeat content with standard chromosome set of both T. fiebrigi and T. angustula (Apidae: Meliponini). Cytogenetic and Genome Research, 163 : pp 52-58. 2023. DOI: [10.1159/000533431 https://doi.org/10.1159/000533431]
Owen, R.E. Chromosome numbers of 15 North American bumble bee species (Hymenoptera, Apidae, Bombini). Canadian Journal of Genetics and Cytology, 25 : pp 26-29. 1983. DOI: [10.1139/g83-004 https://doi.org/10.1139/g83-004]
Barbosa, I.C.O., Schneider, C.H., Goll, L.G., Feldberg, E., Carvalho-Zilse, G.A. Chromosomal mapping of repetitive DNA in Melipona seminigra merrillae Cockerell, 1919 (Hymenoptera, Apidae, Meliponini). Comparative Cytogenetics, 15 : pp 77-87. 2021. DOI: [10.3897/compcytogen.v15.i1.56430 https://doi.org/10.3897/compcytogen.v15.i1.56430]
Nascimento, R.M., Carvalho, A.F., Santana, W.C., Barth, A., Costa, M.A. Karyotype diversity of stingless bees of the genus Frieseomelitta (Hymenoptera, Apidae, Meliponini). Caryologia, 73 : pp 121-126. 2020. DOI: [10.13128/caryologia-610 https://doi.org/10.13128/caryologia-610]
Rocha, M.P., Pompolo, S.G., Dergam, J.A., Fernandes, A., Campos, L.A.O. DNA characterization and karyotypic evolution in the bee genus Melipona (Hymenoptera, Meliponini). Hereditas, 136 : pp 19-27. 2002. DOI: [10.1034/j.1601-5223.2002.1360104.x https://doi.org/10.1034/j.1601-5223.2002.1360104.x]
Owen, R.E., Richards, K.W., Wilkes, A. Chromosome numbers and karyotypic variation in bumble bees (Hymenoptera: Apidae; Bombini). Journal of the Kansas Entomological Society, 68 : pp 290-302. 1995
Brito, R.M., Pompolo, S.G, Magalhães, M.F.M., Barros, E.G., Sakamoto-Hojo, E.T. Cytogenetic characterization of two Partamona species (Hymenoptera, Apinae, Meliponini) by fluorochrome staining and localization of 18S rDNA clusters by FISH. Cytologia, 70 : pp 373-380. 2005. DOI: [10.1508/cytologia.70.373 https://doi.org/10.1508/cytologia.70.373]
Wilfert, L., Gadau, J., Schmid-Hempel, P. A core linkage map of the bumblebee Bombus terrestris. Genome, 49 : pp 1215-1226. 2006. DOI: [10.1139/g06-075 https://doi.org/10.1139/g06-075]
Ramberg, F.B., Kukuk, P., Brown Jr, W.L. Karyotypes of three species of Halictidae (Hymenoptera: Apoidea). Journal of the Kansas Entomological Society, 57 : pp 159-161. 1984
Hoshiba, H., Okada, I., Kusanagi, A. The diploid drone of Apis cerana japonica and its chromosomes. Journal of Apicultural Research, 20 : pp 143-147. 1981. DOI: [10.1080/00218839.1981.11100488 https://doi.org/10.1080/00218839.1981.11100488]
Travenzoli, N.M., Lima, B.A., Cardoso, D.C., Dergam, J.A., Fernandes-Salomão, T.M., Lopes, D.M. Cytogenetic analysis and chromosomal mapping of repetitive DNA in Melipona species (Hymenoptera, Meliponini). Cytogenetic and Genome Research, 158 : pp 213-224. 2019a. DOI: [10.1159/000501754 https://doi.org/10.1159/000501754]
Gonçalvez, G.C., Dalbosco, A.M., Barth, A., Miranda, E.A., Costa, M.A. Comparative cytogenetic analysis of three species of the genus Partamona (Apidae, Meliponini). Apidologie, 52 : pp 80-88. 2020. DOI: [10.1007/s13592-020-00798-7 https://doi.org/10.1007/s13592-020-00798-7]
Travenzoli, N.M., Barbosa, I.C.O., Carvalho-Zilse, G.A., Salomão, T.M.F., Lopes, D.M. Karyotypic description and repetitive DNA chromosome mapping of Melipona interrupta Latreille, 1811 (Hymenoptera: Meliponini). Caryologia, 72 : pp 91-95. 2019b. DOI: [10.13128/cayologia-239 https://doi.org/10.13128/cayologia-239]
Teixeira, G.A., Ferreira, R.P., Lopes, D.M. Comparative cytogenetic analysis reveals chromosomal variability in five stingless bees of the genus Trigona (Apidae, Apinae, Meliponini). Apidologie, 54 : pp 22. 2023. DOI: [10.1007/s13592-023-01002-2 https://doi.org/10.1007/s13592-023-01002-2]
Hoshiba, H. Karyotype and banding analyses on haploid males of the honey bee (Apis mellifera). Proceedings of the Japan Academy Series B, 60 : pp 122-124. 1984a. DOI: [10.2183/pjab.60.122 https://doi.org/10.2183/pjab.60.122]
Bravo, F., Arcos, L. O cariótipo das espécies de Parapartamona (Schwarz) (Hymenoptera, Apidae, Meliponinae) e comentários sobre a sistemática do gênero. Revista Brasileira de Entomologia, 35 : pp 755-759. 1991
Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature, 443 : pp 931-949. 2006. DOI: [10.1038/nature05260 https://doi.org/10.1038/nature05260]
Campos, C.L., Teixeira, G.A., Lopes, D.M., Bitencourt, J.A., Bezerra, D.D., Alves, R.M.O., Werneck, H.A., Waldschmidt, A.M. New patterns of polymorphism in the karyotypic analysis of the genus Plebeia (Hymenoptera, Apidae). Apidologie, 55 : pp 45. 2024. DOI: [10.1007/s13592-024-01090-8 https://doi.org/10.1007/s13592-024-01090-8]
Andrade, B.L.F., Lopes, A.L.G., Teixeira, G.A., Tavares, M.G. Karyotypes and chromosomal mapping of some repetitive DNAs in two stingless bee species (Apidae: Meliponini), with the description of a B chromosome in Plebeia genus. Cytogenetic and Genome Research, 164 : pp 267-275. 2024. DOI: [10.1159/000542295 https://doi.org/10.1159/000542295]
Rocha, M.P., Pompolo, S.G., Campos, L.A.O. Citogenética da tribo Meliponini (Hymenoptera, Apidae). UNESC, Criciúma, : pp 311-320. 2003a
Costa, M.A., Pompolo, S.G., Campos, L.A.O. Supernumerary chromosomes in Partamona cupira (Hymenoptera, Apidae, Meliponinae). Revista Brasileira de Genética, 15 : pp 801-806. 1992
Tosta, V.C., Fernandes-Salomão, T.M., Tavares, M.G., Pompolo, S.G., Barros, E.G., Campos, L.A.O. A RAPD marker associated with B chromosomes in Partamona helleri (Hymenoptera, Apidae). Cytogenetic and Genome Research, 106 : pp 279-283. 2004. DOI: [10.1159/000079299 https://doi.org/10.1159/000079299]
Rocha, M.P., Pompolo, S.G., Fernandes, A., Campos, L.A.O. Melipona: Six decade of cytogenetic. Bioscience Journal, 23 : pp 111-117. 2007
Kerr, W.E. Numbers of chromosomes in some species of bees. Journal of the Kansas Entomological society, 45 : pp 111-122. 1972
Silva, W.R.T., Araújo, E.D., Scher, R. Caracterização do cariótipo de uma população de abelhas Melipona quadrifasciata (Hymenoptera: Meliponini), no município de Brejo Grande/Se. Scientia Plena, 8 : pp 1-6. 2012
Pompolo, S.G., Campos, L.A.O. Karyotypes of two species of stingless bees, Leurotrigona muelleri and Leurotrigona pusilla (Hymenoptera, Meliponinae). Revista Brasileira de Genética, 18 : pp 181-184. 1995
Domingues, A.M.T., Waldschmidt, A.M., Andrade, S.E., Andrade-Souza, V., Alves, R.M.O., Silva Junior, J.C., Costa, M.A. Karyotype characterization of Trigona fulviventris Guérin, 1835 (Hymenoptera, Meliponini) by C banding and fluorochrome staining: Report of a new chromosome number in the genus. Genetics and Molecular Biology, 28 : pp 390-393. 2005. DOI: [10.1590/S1415-47572005000300009 https://doi.org/10.1590/S1415-47572005000300009]
Gregory, T.R., Nicol, J.A., Tamm, H., Kullman, B., Kullman, K., Leitch, I.J., Murray, B.G., Kapraun, D.F., Greilhuber, J., Bennett, M.D. Eukaryotic genome size databases. Nucleic Acids Research, 35 : pp D332-D338. 2007. DOI: [10.1093/nar/gkl828 https://doi.org/10.1093/nar/gkl828]
Elizeu, A.M., Travenzoli, N.M., Ferreira, R.P., Lopes, D.M., Tavares, M.G. Comparative study on the physical mapping of ribosomal genes and repetitive sequences in Friesella schrottkyi (Friese 1900) (Hymenoptera: Apidae, Meliponini). Zoologischer Anzeiger, 292 : pp 225-230. 2021. DOI: [10.1016/j.jcz.2021.04.006 https://doi.org/10.1016/j.jcz.2021.04.006]
Silva, A.A., Rocha, M.P., Pompolo, S.G., Campos, L.A.O., Tavares, M.G. Karyotypic description of the stingless bee Melipona quinquefasciata Lepeletier, 1836 (Hymenoptera, Meliponini) with emphasis on the presence of B chromosomes. Comparative Cytogenetics, 12 : pp 471-482. 2018. DOI: [10.3897/CompCytogen.v12i4.29165 https://doi.org/10.3897/CompCytogen.v12i4.29165]
Tavares, M.G., Ferreira, R.P., Travenzoli, N.M., Lopes, D.M. Karyotypic variation in the stingless bee Trigona spinipes (Hymenoptera: Apidae: Meliponini) from different geographical regions of Brazil. Apidologie, 52 : pp 1358-1367. 2021. DOI: [10.1007/s13592-021-00906-1 https://doi.org/10.1007/s13592-021-00906-1]
Novaes, C.M., Cunha, M.S., Clarindo, W.R., Tosta, V.C., Salomão-Fernades, T.M., Lopes, D.M. Inter- and intra-population B chromosome variability in Partamona helleri (Apidae: Meliponini). Apidologie, 52 : pp 1334-1345. 2021b. DOI: [10.1007/s13592-021-00904-3 https://doi.org/10.1007/s13592-021-00904-3]
Brito, R.M., Costa, M.A., Pompolo, S.G. Characterization and distribution of supernumerary chromosomes in 23 colonies of Partamona helleri (Hymenoptera, Apidae, Meliponinae). Brazilian Journal of Genetics, 20 : pp 185-188. 1997
Maffei, E.M.D., Pompolo, S.G., Silva-Jr, J.C., Caixeiro, A.P.A., Rocha, M.P., Dergam, J.A. Silver staining of nucleolar organizer regions (NORs) in some species of Hymenoptera (bees and parasitic wasps) and Coleoptera (lady beetle). Cytobios, 104 : pp 119-125. 2001
Martins, C.C.C., Diniz, D., Sobrinho-Scudeler, P.E., Foresti, F., Campos, L.A.O., Costa, M.A. Investigation of Partamona helleri (Apidae, Meliponini) B chromosome origin. An approach by microdissection and whole chromosome painting. Apidologie, 44 : pp 75-81. 2013. DOI: [10.1007/s13592-012-0157-6 https://doi.org/10.1007/s13592-012-0157-6]
Lopes, D.M., Carvalho, C.R., Clarindo, W.R., Praça, M.M., Tavares, M.G. Genome size estimation of three stingless bee species (Hymenoptera, Meliponinae) by flow cytometry. Apidologie, 40 : pp 517-523. 2009. DOI: [10.1051/apido/2009030 https://doi.org/10.1051/apido/2009030]
Sahara, K., Marec, F., Traut, W. TTAGG telomeric repeats in chromosomes of some insects and other arthropods. Chromosome Research, 7 : pp 449-460. 1999. DOI: [10.1023/A:1009297729547 https://doi.org/10.1023/A:1009297729547]
Martins, C.C.C., Duarte, O.M.P., Waldschmidt, A.M., Alves, R.M.O., Costa, M.A. New occurrence of B chromosomes in Partamona helleri (Friese, 1900) (Hymenoptera, Meliponini). Genetics and Molecular Biology, 32 : pp 782-785. 2009. DOI: [10.1590/S1415-47572009005000065 https://doi.org/10.1590/S1415-47572009005000065]
Stolle, E., Wilfert, L., Schmid-Hempel, R., Schmid-Hempel, P., Kube, M., Reinhardt, R., Moritz, R.F. A second generation genetic map of the bumblebee Bombus terrestris (Linnaeus, 1758) reveals slow genome and chromosome evolution in the Apidae. BMC Genomics, 12 : pp 48. 2011. DOI: [10.1186/1471-2164-12-48 https://doi.org/10.1186/1471-2164-12-48]
Ayabe, T., Hoshiba, H., Ono, M. Cytological evidence for triploid males and females in the bumblebee, Bombus terrestris. Chromosome research, 12 : pp 215-223. 2004. DOI: [10.1023/B:CHRO.0000021880.83639.4b https://doi.org/10.1023/B:CHRO.0000021880.83639.4b]
Krinski, D., Fernandes, A., Rocha, M.P., Pompolo, S.G. Karyotypic description of the stingless bee Oxytrigona cf. flaveola (Hymenoptera, Apidae, Meliponina) of a colony from Tangará da Serra, Mato Grosso State, Brazil. Genetics and Molecular Biology, 33 : pp 494-498. 2010. DOI: [10.1590/S1415-47572010000300020 https://doi.org/10.1590/S1415-47572010000300020]
